Loss of chloride channel ClC-5 impairs endocytosis by defective trafficking of megalin and cubilin in kidney proximal tubules
- PMID: 12815097
- PMCID: PMC166253
- DOI: 10.1073/pnas.1432873100
Loss of chloride channel ClC-5 impairs endocytosis by defective trafficking of megalin and cubilin in kidney proximal tubules
Abstract
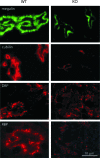
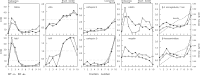
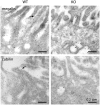
Loss of the renal endosome-associated chloride channel, ClC-5, in Dent's disease and knockout (KO) mice strongly inhibits endocytosis of filtered proteins by kidney proximal tubular cells (PTC). The underlying mechanism remains unknown. We therefore tested whether this endocytic failure could primarily reflect a loss of reabsorption by the multiligand receptors, megalin, and cubilin, caused by a trafficking defect. Impaired protein endocytosis in PTC of ClC-5 KO mice was demonstrated by (i) a major decreased uptake of injected 125I-beta 2-microglobulin, but not of the fluid-phase tracer, FITC-dextran, (ii) reduced labeling of endosomes by injected peroxidase and for the endogenous megalin/cubilin ligands, vitamin D- and retinol-binding proteins, and (iii) urinary appearance of low-molecular-weight proteins and the selective cubilin ligand, transferrin. Contrasting with preserved mRNA levels, megalin and cubilin abundance was significantly decreased in kidney extracts of KO mice. Percoll gradients resolving early and late endosomes (Rab5a, Rab7), brush border (villin, aminopeptidase M), and a dense peak comprising lysosomes (acid hydrolases) showed a disappearance of the brush border component for megalin and cubilin in KO mice. Quantitative ultrastructural immunogold labeling confirmed the overall decrease of megalin and cubilin in PTC and their selective loss at the brush border. In contrast, total contents of the rate-limiting endocytic catalysts, Rab5a and Rab7, were unaffected. Thus, impaired protein endocytosis caused by invalidation of ClC-5 primarily reflects a trafficking defect of megalin and cubilin in PTC.
Figures

References
-
- Christensen, E. I. & Birn, H. (2002) Nat. Rev. Mol. Cell Biol. 3 256-266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

