Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor
- PMID: 12815188
- PMCID: PMC2343223
- DOI: 10.1113/jphysiol.2003.039990
Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor
Abstract
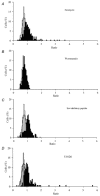
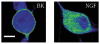
Nerve growth factor (NGF) causes a rapid sensitisation of nociceptive sensory neurones to painful thermal stimuli owing to an action on the heat and capsaicin receptor TRPV1 (formerly known as VR1). We have developed a new technique to study this rapid sensitisation of TRPV1 by monitoring the effects of NGF on the increase in intracellular calcium concentration ([Ca2+]i) following exposure to capsaicin. Brief applications of capsaicin caused a rise in [Ca2+]i, and NGF was found to enhance this rise in 37 % of capsaicin-responsive neurones within 2 min. Pathways responsible for transducing the sensitisation of TRPV1 by TrkA, the NGF receptor, were characterised by observing the effects of inhibitors of key members of NGF-activated second messenger signalling cascades. Specific inhibitors of the ras/MEK (mitogen-activated protein and extracellular signal-regulated kinases) pathway and of phospholipase C did not abolish the NGF-induced sensitisation, but wortmannin, a specific inhibitor of phosphatidylinositol-3-kinase (PI3K), totally abolished the effect of NGF. Pharmacological blockade of protein kinase C (PKC) or calcium-calmodulin-dependent protein kinase II (CaMK II) activation also prevented NGF-induced sensitisation, while blockade of protein kinase A (PKA) was without effect. These data indicate that the crucial early pathway activated by NGF involves PI3K, while PKC and CaMK II are also involved, probably at subsequent stages of the NGF-activated signalling pathway.
Figures





References
-
- Bergmann I, Reiter R, Toyka KV, Koltzenburg M. Nerve growth factor evokes hyperalgesia in mice lacking the low-affinity neurotrophin receptor p75. Neurosci Lett. 1998;255:87–90. - PubMed
-
- Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW. cAMP-dependent protein kinase regulates desensitisation of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. - PubMed
-
- Bron R, Klesse LJ, Shah K, Parada LF, Winter J. Activation of Ras is necessary and sufficient for upregulation of vanilloid receptor type 1 in sensory neurons by neurotrophic factors. Mol Cell Neurosci. 2003;22:118–132. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

