Voltage-gated K+ channels in rat small cerebral arteries: molecular identity of the functional channels
- PMID: 12815189
- PMCID: PMC2343285
- DOI: 10.1113/jphysiol.2003.040014
Voltage-gated K+ channels in rat small cerebral arteries: molecular identity of the functional channels
Abstract
Voltage-gated potassium (KV) channels represent an important dilator influence in the cerebral circulation, but the composition of these tetrameric ion channels remains unclear. The goals of the present study were to evaluate the contribution of KV1 family channels to the resting membrane potential and diameter of small rat cerebral arteries, and to identify the alpha-subunit composition of these channels using patch-clamp, molecular and immunological techniques. Initial studies indicated that 1 micromol l(-1) correolide (COR), a specific antagonist of KV1 channels, depolarized vascular smooth muscle cells (VSMCs) in pressurized (60 mmHg) cerebral arteries from -55 +/- 1 mV to -34 +/- 1 mV, and reduced the resting diameter from 152 +/- 15 microm to 103 +/- 20 microm. In patch clamped VSMCs from these arteries, COR-sensitive KV1 current accounted for 65 % of total outward KV current and was observed at physiological membrane potentials. RT-PCR identified mRNA encoding each of the six classical KV1 alpha-subunits, KV1.1-1.6, in rat cerebral arteries. However, only the KV1.2 and 1.5 proteins were detected by Western blot. The expression of these proteins in VSMCs was confirmed by immunocytochemistry and co-immunoprecipitation of KV1.2 and 1.5 from VSMC membranes suggested KV1.2/1.5 channel assembly. Subsequently, the pharmacological and voltage-sensitive properties of KV1 current in VSMCs were found to be consistent with a predominant expression of KV1.2/1.5 heterotetrameric channels. The findings of this study suggest that KV1.2/1.5 heterotetramers are preferentially expressed in rat cerebral VSMCs, and that these channels contribute to the resting membrane potential and diameter of rat small cerebral arteries.
Figures



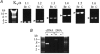
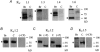

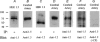

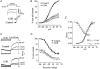
References
-
- Aiello EA, Walsh MP, Cole WC. Phosphorylation by protein kinase A enhances delayed rectifier K+ current in rabbit vascular smooth muscle cells. Am J Physiol. 1995;268:H926–934. - PubMed
-
- Archer SL, Souil E, Dinh-Xuan AT, Schremmer B, Mercier J-C, El Yaagoubi A, Nguyen-Huu L, Reeve HL, Hampl V. Molecular identification of the role of voltage-gated K+ channels, Kv1. 5 and Kv2.1, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. J Clin Invest. 1998;101:2319–2330. - PMC - PubMed
-
- Attali B, Lesage F, Ziliani P, Guillemare E, Honore E, Waldmann R, Hugnot J-P, Mattel M-G, Lazdunski M, Barhanin J. Multiple mRNA isoforms encoding the mouse cardiac Kv1–5 delayed rectifier K+ channel. J Biol Chem. 1993;268:24283–24289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

