Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules
- PMID: 12819012
- PMCID: PMC1868188
- DOI: 10.1016/S0002-9440(10)63631-0
Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules
Abstract
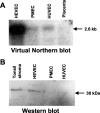
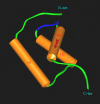
Lymphocyte homing to secondary lymphoid tissue and lesions of chronic inflammation is directed by multi-step interactions between the circulating cells and the specialized endothelium of high endothelial venules (HEVs). In this study, we used the PCR-based method of suppression subtractive hybridization (SSH) to identify novel HEV genes by comparing freshly purified HEV endothelial cells (HEVECs) with nasal polyp-derived microvascular endothelial cells (PMECs). By this approach, we cloned the first nuclear factor preferentially expressed in HEVECs, designated nuclear factor from HEVs (NF-HEV). Virtual Northern and Western blot analyses showed strong expression of NF-HEV in HEVECs, compared to human umbilical vein endothelial cells (HUVECs) and PMECs. In situ hybridization and immunohistochemistry revealed that NF-HEV mRNA and protein are expressed at high levels and rather selectively by HEVECs in human tonsils, Peyers's patches, and lymph nodes. The NF-HEV protein was found to contain a bipartite nuclear localization signal, and was targeted to the nucleus when ectopically expressed in HUVECs and HeLa cells. Furthermore, endogenous NF-HEV was found in situ to be confined to the nucleus of tonsillar HEVECs. Finally, threading and molecular modeling studies suggested that the amino-terminal part of NF-HEV (aa 1-60) corresponds to a novel homeodomain-like Helix-Turn-Helix (HTH) DNA-binding domain. Similarly to the atypical homeodomain transcription factor Prox-1, which plays a critical role in the induction of the lymphatic endothelium phenotype, NF-HEV may be one of the key nuclear factors that controls the specialized HEV phenotype.
Figures




References
-
- Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM: Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 1998, 91:3527-3561 - PubMed
-
- Risau W: Differentiation of endothelium. EMBO J 1995, 9:926-933 - PubMed
-
- Girard JP, Springer TA: High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol Today 1995, 16:449-457 - PubMed
-
- Kraal G, Mebius RE: High endothelial venules: lymphocyte traffic control and controlled traffic. Adv Immunol 1997, 65:347-395 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

