Mononuclear leukocytes bind to specific hyaluronan structures on colon mucosal smooth muscle cells treated with polyinosinic acid:polycytidylic acid: inter-alpha-trypsin inhibitor is crucial to structure and function
- PMID: 12819017
- PMCID: PMC1868154
- DOI: 10.1016/s0002-9440(10)63636-x
Mononuclear leukocytes bind to specific hyaluronan structures on colon mucosal smooth muscle cells treated with polyinosinic acid:polycytidylic acid: inter-alpha-trypsin inhibitor is crucial to structure and function
Abstract
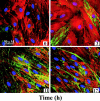
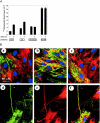
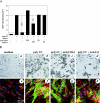
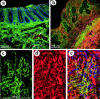
Inflammatory bowel disease (IBD) is a chronic disorder whose etiology is linked to triggering events, including viral infections, that lead to immunoregulatory dysfunction in genetically susceptible people. Characteristic pathological changes include increased mononuclear leukocyte influx into the intestinal mucosa as well as mucosal smooth muscle cell (M-SMC) hyperplasia. Virus infection or viral mimic [polyinosinic acid:polycytidylic acid (polyI:C)] treatment of human colon M-SMCs in vitro increases cell surface hyaluronan (HA), and nonactivated mononuclear leukocytes bind to virus-induced HA structures by interactions that involve the HA-binding receptor CD44. In this study, confocal microscopy reveals increased HA on poly I:C-treated M-SMC surfaces within 3 hours, arrayed in coat-like structures. By 17 hours, novel, lengthy cable structures are evident, and these are primarily responsible for mediating leukocyte adhesion. Immunohistochemical staining demonstrates components of the inter-alpha-trypsin inhibitor (IalphaI) complex in both coat-like and cable structures. M-SMCs co-treated with polyI:C and a polyclonal antibody to IalphaI display HA in coats but with diminished cables, and they bind significantly fewer leukocytes than M-SMCs treated with polyI:C alone. Western blot data suggest that heavy chains of IalphaI are specifically associated with cable structures. Staining of tissue sections from patients with IBD demonstrates the presence of HA in inflamed colon tissue, and shows that HA-associated IalphaI staining increases in the mucosa of inflamed IBD specimens compared to noninflamed sections from the same patient, establishing a probable link between the observations in vitro and the progression of the inflammatory process in IBD.
Figures




References
-
- Kangro HO, Chong SK, Hardiman A, Heath RB, Walker-Smith JA: A prospective study of virus and mycoplasm infections in chronic inflammatory bowel disease. Gastroenterology 1990, 98:549-553 - PubMed
-
- Yanai H, Shimizu N, Nagasaki S, Mitani N, Okita K: Epstein-Barr virus infection of the colon with inflammatory bowel disease. Am J Gastroenterology 1999, 98:549-553 - PubMed
-
- de la Motte CA, Hascall VC, Calabro A, Yen-Lieberman B, Strong SA: Mononuclear leukocytes preferentially bind via CD44 to hyaluronan on human intestinal mucosal smooth muscle cells after virus infection or treatment with poly I: C. J Biol Chem 1999, 274:30747-30755 - PubMed
-
- Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B: CD44 is the principal cell surface receptor for hyaluronate. Cell 1990, 61:1303-1313 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

