Expression profiling of cytokines and related genes in regenerating skeletal muscle after cardiotoxin injection: a role for osteopontin
- PMID: 12819025
- PMCID: PMC1868192
- DOI: 10.1016/S0002-9440(10)63644-9
Expression profiling of cytokines and related genes in regenerating skeletal muscle after cardiotoxin injection: a role for osteopontin
Abstract
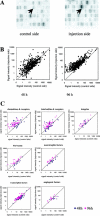
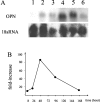
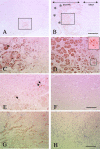
To examine the roles of cytokines in muscle regeneration, we injected cardiotoxin into mouse tibialis anterior muscle and examined the expression profiles of cytokines and related genes in the regeneration process. Expression of 40, 64, and 7 genes among 522 genes spotted on a cytokine expression array were increased more than fivefold at 48 hours, 96 hours, and 7 days after toxin injection, respectively, when compared with those of the control muscle. Especially the levels of mRNA for chemokines and chemokine receptors, many of which are potent regulators of macrophages, were highly elevated 48 hours after injury. The expression of osteopontin (OPN), a versatile regulator of inflammation and tissue repair, was up-regulated more than 118-fold in regenerating muscle at 48 hours after injury. Northern blotting confirmed that the expression of OPN was highest at 48 hours after cardiotoxin injection and declined sharply thereafter. Immunohistochemistry showed that OPN was detected both in the cytoplasm of macrophages and in necrotic muscle infiltrated with macrophages. Our studies suggest OPN may serve as an adhesion molecule that promotes macrophage binding to necrotic fibers and may be an important mediator in the early phase of muscle regeneration.
Figures


Similar articles
-
Heat stress facilitates the regeneration of injured skeletal muscle in rats.J Orthop Sci. 2007 Jan;12(1):74-82. doi: 10.1007/s00776-006-1083-0. Epub 2007 Jan 31. J Orthop Sci. 2007. PMID: 17260121
-
Serum Osteopontin as a Novel Biomarker for Muscle Regeneration in Duchenne Muscular Dystrophy.Am J Pathol. 2016 May;186(5):1302-12. doi: 10.1016/j.ajpath.2016.01.002. Epub 2016 Mar 8. Am J Pathol. 2016. PMID: 26963343
-
Satellite cells produce neural chemorepellent semaphorin 3A upon muscle injury.Anim Sci J. 2013 Feb;84(2):185-9. doi: 10.1111/asj.12014. Epub 2012 Dec 17. Anim Sci J. 2013. PMID: 23384361
-
Myotoxic phospholipases A2 and the regeneration of skeletal muscles.Toxicon. 2003 Dec 15;42(8):933-45. doi: 10.1016/j.toxicon.2003.11.011. Toxicon. 2003. PMID: 15019492 Review.
-
Effects of the immune system on muscle regeneration.Curr Top Dev Biol. 2024;158:239-251. doi: 10.1016/bs.ctdb.2024.01.013. Epub 2024 Apr 8. Curr Top Dev Biol. 2024. PMID: 38670708 Review.
Cited by
-
Cobra CRISP functions as an inflammatory modulator via a novel Zn2+- and heparan sulfate-dependent transcriptional regulation of endothelial cell adhesion molecules.J Biol Chem. 2010 Nov 26;285(48):37872-83. doi: 10.1074/jbc.M110.146290. Epub 2010 Oct 2. J Biol Chem. 2010. PMID: 20889969 Free PMC article.
-
Translating golden retriever muscular dystrophy microarray findings to novel biomarkers for cardiac/skeletal muscle function in Duchenne muscular dystrophy.Pediatr Res. 2016 Apr;79(4):629-36. doi: 10.1038/pr.2015.257. Epub 2015 Dec 16. Pediatr Res. 2016. PMID: 26672735 Free PMC article.
-
Reversal of deficits in aged skeletal muscle during disuse and recovery in response to treatment with a secrotome product derived from partially differentiated human pluripotent stem cells.Geroscience. 2021 Dec;43(6):2635-2652. doi: 10.1007/s11357-021-00423-0. Epub 2021 Aug 24. Geroscience. 2021. PMID: 34427856 Free PMC article.
-
Failure to resolve inflammation contributes to juvenile onset cardiac damage in a mouse model of Duchenne Muscular Dystrophy.bioRxiv [Preprint]. 2025 Jan 29:2024.08.15.607998. doi: 10.1101/2024.08.15.607998. bioRxiv. 2025. Update in: Cell Death Dis. 2025 Jul 9;16(1):505. doi: 10.1038/s41419-025-07816-5. PMID: 39185176 Free PMC article. Updated. Preprint.
-
The chemokine system in arteriogenesis and hind limb ischemia.J Vasc Surg. 2007 Jun;45 Suppl A(Suppl A):A48-56. doi: 10.1016/j.jvs.2007.02.030. J Vasc Surg. 2007. PMID: 17544024 Free PMC article. Review.
References
-
- Bischoff R: The satellite cell and muscle regeneration. Engel AG Franzini-Armstrong C eds. Myology. 1994:pp 97-118 McGraw-Hill, New York
-
- Best TM, Hunter KD: Muscle injury and repair. Phys Med Rehabil Clin North Am 2000, 11:251-266 - PubMed
-
- Grounds MD: Age-associated changes in the response of skeletal muscle cells to exercise and regeneration. Ann NY Acad Sci 1998, 854:78-91 - PubMed
-
- Grounds MD: Muscle regeneration: molecular aspects and therapeutic implications. Curr Opin Neurol 1999, 12:535-543 - PubMed
-
- Seale P, Rudnicki MA: A new look at the origin, function, and “stem cell” status of muscle satellite cells. Dev Biol 2000, 218:115-124 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

