Activation of Src kinase in platelet-derived growth factor-B-dependent tubular regeneration after acute ischemic renal injury
- PMID: 12819032
- PMCID: PMC1868189
- DOI: 10.1016/S0002-9440(10)63651-6
Activation of Src kinase in platelet-derived growth factor-B-dependent tubular regeneration after acute ischemic renal injury
Abstract
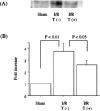
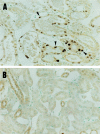
We previously reported that the platelet-derived growth factor B-chain (PDGF-B)/PDGF receptor (PDGFR) axis is involved in tubular regeneration after ischemia/reperfusion injury of the kidney. In the present study, we examined the activation of Src tyrosine kinase, a crucially important signaling molecule for PDGFR, and assessed the role of Src in PDGF-B-dependent renal tubular regeneration afterischemia/reperfusion injury. Immunoblot using clone 28, a monoclonal antibody specific for the active form of Src kinases, demonstrated increased active Src expression in the injured rat kidney 6 hours after reperfusion with peak activation at 12 hours. In vitro kinase assay confirmed increased Src activity that concurred with PDGFR-beta activation as detected by the increment of receptor-phosphorylated tyrosine. Immunohistochemistry using clone 28 demonstrated that active Src was preferentially expressed in the S3 segment of the proximal tubule in reperfused kidney, where it is not normally expressed. This enhanced expression of active Src was co-localized with the increased PDGFR expression in the tubular cells that were undergoing cell proliferation cycle. Trapidil administration suppressed Src and PDGFR-beta activation in the reperfused kidney and resulted in deteriorated renal function. These findings suggest that active Src participates in PDGF-B-dependent regeneration of tubular cells from acute ischemic injury.
Figures






Similar articles
-
Role of PDGF B-chain and PDGF receptors in rat tubular regeneration after acute injury.Am J Pathol. 1999 Nov;155(5):1689-99. doi: 10.1016/S0002-9440(10)65484-3. Am J Pathol. 1999. PMID: 10550325 Free PMC article.
-
Expression of platelet-derived growth factor (PDGF) in the epididymis and analysis of the epididymal development in PDGF-A, PDGF-B, and PDGF receptor beta deficient mice.Biol Reprod. 2004 Jan;70(1):168-77. doi: 10.1095/biolreprod.103.019232. Epub 2003 Oct 1. Biol Reprod. 2004. PMID: 14522834
-
The role of platelet-derived growth factor signaling in healing myocardial infarcts.J Am Coll Cardiol. 2006 Dec 5;48(11):2315-23. doi: 10.1016/j.jacc.2006.07.060. Epub 2006 Nov 13. J Am Coll Cardiol. 2006. PMID: 17161265
-
Bone marrow plasticity revisited: protection or differentiation in the kidney tubule?J Clin Invest. 2005 Jul;115(7):1705-8. doi: 10.1172/JCI25540. J Clin Invest. 2005. PMID: 16007248 Free PMC article. Review.
-
The role of PDGF-B/PDGFR-BETA axis in the normal development and carcinogenesis of the breast.Crit Rev Oncol Hematol. 2018 Nov;131:46-52. doi: 10.1016/j.critrevonc.2018.08.002. Epub 2018 Aug 28. Crit Rev Oncol Hematol. 2018. PMID: 30293705 Review.
Cited by
-
Potential targeted therapy and diagnosis based on novel insight into growth factors, receptors, and downstream effectors in acute kidney injury and acute kidney injury-chronic kidney disease progression.Signal Transduct Target Ther. 2020 Feb 14;5(1):9. doi: 10.1038/s41392-020-0106-1. Signal Transduct Target Ther. 2020. PMID: 32296020 Free PMC article. Review.
-
SLURP-1 Controls Growth and Migration of Lung Adenocarcinoma Cells, Forming a Complex With α7-nAChR and PDGFR/EGFR Heterodimer.Front Cell Dev Biol. 2021 Sep 14;9:739391. doi: 10.3389/fcell.2021.739391. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34595181 Free PMC article.
-
A small molecule fibrokinase inhibitor in a model of fibropolycystic hepatorenal disease.World J Nephrol. 2018 Sep 7;7(5):96-107. doi: 10.5527/wjn.v7.i5.96. World J Nephrol. 2018. PMID: 30211028 Free PMC article.
-
Activation of Src mediates PDGF-induced Smad1 phosphorylation and contributes to the progression of glomerulosclerosis in glomerulonephritis.PLoS One. 2011 Mar 22;6(3):e17929. doi: 10.1371/journal.pone.0017929. PLoS One. 2011. PMID: 21445358 Free PMC article.
-
EGFR activity is required for renal tubular cell dedifferentiation and proliferation in a murine model of folic acid-induced acute kidney injury.Am J Physiol Renal Physiol. 2013 Feb 15;304(4):F356-66. doi: 10.1152/ajprenal.00553.2012. Epub 2012 Dec 19. Am J Physiol Renal Physiol. 2013. PMID: 23255615 Free PMC article.
References
-
- Bacallo R, Fine LG: Molecular events in the organization of renal tubular epithelium from nephrogenesis to regeneration. Am J Physiol 1989, 257:F913-F924 - PubMed
-
- Wallin A, Zhang G, Jones TW, Jaken S, Stevens JL: Mechanism of the nephrogenic repair response: studies on proliferation and vimentin expression after 35S-(1,2-dichlorovinyl)-L-cysteine nephrotoxicity in vivo and in cultured proximal tubule epithelial cells. Lab Invest 1992, 66:474-484 - PubMed
-
- Hammerman MR: Growth factors and apoptosis in acute renal injury. Curr Opin Nephrol Hypertens 1998, 7:419-424 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

