Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation
- PMID: 12819035
- PMCID: PMC1868171
- DOI: 10.1016/S0002-9440(10)63654-1
Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation
Abstract
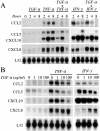
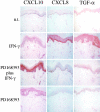
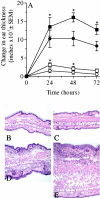
During inflammatory skin disorders such as psoriasis, atopic dermatitis, and allergic contact dermatitis, epidermal keratinocytes overexpress large amounts of soluble epidermal growth factor receptor ligands in response to tumor necrosis factor alpha and interferon gamma. These cytokines also promote de novo synthesis of numerous chemokines, including CCL2/MCP-1, CCL5/RANTES, CXCL10/IP-10, and CXCL8/IL-8, in turn responsible for the recruitment of different leukocyte populations. This study demonstrates that stimulation of EGFR down-regulates CCL2, CCL5, and CXCL10, while it increases CXCL8 expression in keratinocytes. Conversely, EGFR signaling blockade produces opposite effects, with increased CCL2, CCL5, and CXCL10, and reduced CXCL8 expression. In a mouse model of contact hypersensitivity, a single topical administration of a selective EGFR kinase blocker before antigen challenge results in a markedly enhanced immune response with increased chemokine expression and heavier inflammatory cell infiltrate. Targeting EGFR on epithelial cells may thus have profound impact on inflammatory and immune responses.
Figures







References
-
- Sunnarborg SW, Hinkle CL, Stevenson M, Russell WE, Raska CS, Peschon JJ, Castner BJ, Gerhart MJ, Paxton RJ, Black RA, Lee DC: Tumor necrosis factor-α converting enzyme (TACE) regulates epidermal growth factor receptor ligand availability. J Biol Chem 2002, 277:12838-12845 - PubMed
-
- Schlessinger J: Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell 2002, 110:669-672 - PubMed
-
- Peus D, Hamacher L, Pittelkow MR: EGF-receptor tyrosine kinase inhibition induces keratinocyte growth arrest and terminal differentiation. J Invest Dermatol 1997, 109:751-756 - PubMed
-
- Hansen LA, Woodson RL, Holbus S, Strain K, Lo YC, Yuspa SH: The epidermal growth factor receptor is required to maintain the proliferative population in the basal compartment of epidermal tumors. Cancer Res 2000, 60:3328-3332 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

