Acute tubular injury causes dysregulation of cellular cholesterol transport proteins
- PMID: 12819036
- PMCID: PMC1868170
- DOI: 10.1016/S0002-9440(10)63655-3
Acute tubular injury causes dysregulation of cellular cholesterol transport proteins
Abstract
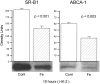
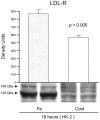
Acute renal injury causes accumulation of free and esterified cholesterol (FC, CE) in proximal tubules, mediated, at least in part, by increased cholesterol synthesis. Normally, this would trigger compensatory mechanisms such as increased efflux and decreased influx to limit or reverse the cholesterol overload state. This study sought to determine the integrity of these compensatory pathways following acute renal damage. Rhabdomyolysis-induced acute renal failure was induced in mice by glycerol injection. Normal mice served as controls. After 18 hours, BUN levels and renal cortical FC/CE content were determined. Expression of ABCA-1 and SR-B1 (cholesterol efflux proteins) were assessed by Western blot. Renal cortical LDL receptor (LDL-R; a cholesterol importer) regulation was gauged by quantifying its mRNA. To obtain proximal tubule cell-specific data, the impact of oxidant (Fe) stress on cultured HK-2 cell LDL-R, SR-B1, and ABCA-1 proteins and their mRNAs (versus controls) was assessed. Glycerol evoked marked azotemia and striking FC/CE increments (44%, 384%, respectively). Paradoxically, renal cortical SR-B1 and ABCA-1 protein reductions and LDL-R mRNA increments resulted. Fe-induced injury suppressed HK-2 cell SR-B1, ABCA-1, and their mRNAs. LDL-R protein rose with the in vitro Fe challenge. Renal tubular cell injury causes dysregulation of SR-B1, ABCA-1, and LDL-R protein expression, changes which should contribute to a cholesterol overload state. Reductions in HK-2 cell SR-B1 and ABCA-1 mRNAs and increases in renal cortical LDL-R mRNA imply that this dysregulation reflects, at least in part, altered genomic/transcriptional events.
Figures
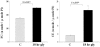


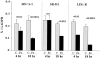
Similar articles
-
Experimental glomerulopathy alters renal cortical cholesterol, SR-B1, ABCA1, and HMG CoA reductase expression.Am J Pathol. 2003 Jan;162(1):283-91. doi: 10.1016/S0002-9440(10)63819-9. Am J Pathol. 2003. PMID: 12507911 Free PMC article.
-
Sepsis syndrome stimulates proximal tubule cholesterol synthesis and suppresses the SR-B1 cholesterol transporter.Kidney Int. 2003 Jan;63(1):123-33. doi: 10.1046/j.1523-1755.2003.00735.x. Kidney Int. 2003. PMID: 12472775
-
Proximal tubular cholesterol loading after mitochondrial, but not glycolytic, blockade.Am J Physiol Renal Physiol. 2003 Dec;285(6):F1092-9. doi: 10.1152/ajprenal.00187.2003. Epub 2003 Sep 2. Am J Physiol Renal Physiol. 2003. PMID: 12952856
-
Foam cells in atherosclerosis.Clin Chim Acta. 2013 Sep 23;424:245-52. doi: 10.1016/j.cca.2013.06.006. Epub 2013 Jun 16. Clin Chim Acta. 2013. PMID: 23782937 Review.
-
[Molecular mechanism of reverse cholesterol transport].Nihon Rinsho. 2001 Feb;59 Suppl 2:463-7. Nihon Rinsho. 2001. PMID: 11351631 Review. Japanese. No abstract available.
Cited by
-
Renal mass reduction results in accumulation of lipids and dysregulation of lipid regulatory proteins in the remnant kidney.Am J Physiol Renal Physiol. 2009 Jun;296(6):F1297-306. doi: 10.1152/ajprenal.90761.2008. Epub 2009 Apr 8. Am J Physiol Renal Physiol. 2009. PMID: 19357177 Free PMC article.
-
Atherosclerosis in chronic kidney disease: the role of macrophages.Nat Rev Nephrol. 2011 Jan;7(1):45-54. doi: 10.1038/nrneph.2010.157. Epub 2010 Nov 23. Nat Rev Nephrol. 2011. PMID: 21102540 Free PMC article. Review.
-
Metabolic effects of RUBCN/Rubicon deficiency in kidney proximal tubular epithelial cells.Autophagy. 2020 Oct;16(10):1889-1904. doi: 10.1080/15548627.2020.1712107. Epub 2020 Jan 16. Autophagy. 2020. PMID: 31944172 Free PMC article.
-
Anthocyanins inhibit high-glucose-induced cholesterol accumulation and inflammation by activating LXRα pathway in HK-2 cells.Drug Des Devel Ther. 2015 Sep 4;9:5099-113. doi: 10.2147/DDDT.S90201. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26379423 Free PMC article.
-
Growth and development alter susceptibility to acute renal injury.Kidney Int. 2008 Sep;74(5):674-8. doi: 10.1038/ki.2008.251. Epub 2008 Jun 18. Kidney Int. 2008. PMID: 18563055 Free PMC article.
References
-
- Zager RA, Johnson A: Renal cortical cholesterol accumulation is an integral component of the systemic stress response. Kidney Int 2001, 60:2299-2310 - PubMed
-
- Zager RA, Burkhart KM, Johnson AC, Sacks BM: Increased proximal tubular cholesterol content: implications for cell injury and “acquired cytoresistance”. Kidney Int 1999, 56:1788-1797 - PubMed
-
- Zager RA: Plasma membrane cholesterol: a critical determinant of cellular energetics and tubular resistance to attack. Kidney Int 2000, 58:193-205 - PubMed
-
- Zager RA, Johnson A, Anderson K, Wright S: Cholesterol ester accumulation: an immediate consequence of acute in vivo ischemic renal injury. Kidney Int 2001, 59:1750-1761 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

