MYCN enhances P-gp/MDR1 gene expression in the human metastatic neuroblastoma IGR-N-91 model
- PMID: 12819037
- PMCID: PMC1868150
- DOI: 10.1016/S0002-9440(10)63656-5
MYCN enhances P-gp/MDR1 gene expression in the human metastatic neuroblastoma IGR-N-91 model
Abstract
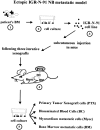
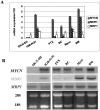
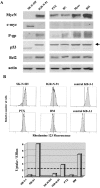
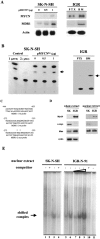
Despite intensive high-dose chemotherapy and autologous hematopoietic stem cell transplantation, disseminated neuroblastoma (NB) frequently proves to be chemosensitive but not chemocurable, and more often so in NB-presenting MYCN amplification. To assess the direct relationship between the MYCN oncogene and chemoresistance acquisition during NB metastatic dissemination, we have studied MYCN and MDR1 genes using the human IGR-N-91 ectopic xenograft metastatic model. This characterized experimental in vitro model includes human neuroblasts derived from a subcutaneous primary tumor xenograft, disseminated blood cells, myocardium, and bone marrow (BM) metastatic cells. All IGR-N-91-derived neuroblasts harbor a consistent MYCN genomic content but, unlike primary tumor xenograft, BM, and myocardium, human neuroblasts elicit a concomitant increase in MYCN and MDR1 transcripts levels, consistent with chemoresistance phenotype and active P-gp. In contrast, no variation of MRP1 transcript level was associated with the metastatic process in this model. Using an MDR1 promoter-CAT construct, we have shown that the MycN protein activates MDR1 transcription both in exogenous transient MYCN-transfected SK-N-SH cells and in endogenous BM metastatic neuroblasts with an increase in the MYCN transcript level. Band-shift experiments indicate that IGR-N-91 cells enriched with the MycN transcription factor do bind to two E-box motifs localized within the MDR1 promoter. Overall, our data indicate that MYCN overexpression increment contributes to the acquired drug resistance that occurs throughout the NB metastatic process.
Figures

Similar articles
-
Coactivation of the MDR1 and MYCN genes in human neuroblastoma cells during the metastatic process in the nude mouse.Cancer Res. 1994 Apr 15;54(8):2256-61. Cancer Res. 1994. PMID: 8174136
-
MYCN-mediated regulation of the MRP1 promoter in human neuroblastoma.Oncogene. 2004 Jan 22;23(3):753-62. doi: 10.1038/sj.onc.1207151. Oncogene. 2004. PMID: 14737110
-
Oncogene MYCN regulates localization of NKT cells to the site of disease in neuroblastoma.J Clin Invest. 2007 Sep;117(9):2702-12. doi: 10.1172/JCI30751. J Clin Invest. 2007. PMID: 17710228 Free PMC article.
-
The MYCN oncoprotein as a drug development target.Cancer Lett. 2003 Jul 18;197(1-2):125-30. doi: 10.1016/s0304-3835(03)00096-x. Cancer Lett. 2003. PMID: 12880971 Review.
-
Role of the MRP1/ABCC1 multidrug transporter protein in cancer.IUBMB Life. 2007 Dec;59(12):752-7. doi: 10.1080/15216540701736285. IUBMB Life. 2007. PMID: 18085475 Review.
Cited by
-
MDR1 mediated chemoresistance: BMI1 and TIP60 in action.Biochim Biophys Acta. 2016 Aug;1859(8):983-93. doi: 10.1016/j.bbagrm.2016.06.002. Epub 2016 Jun 22. Biochim Biophys Acta. 2016. PMID: 27295567 Free PMC article.
-
P-glycoprotein: new insights into structure, physiological function, regulation and alterations in disease.Heliyon. 2022 Jun 22;8(6):e09777. doi: 10.1016/j.heliyon.2022.e09777. eCollection 2022 Jun. Heliyon. 2022. PMID: 35789865 Free PMC article. Review.
-
Lapatinib potentiates cytotoxicity of YM155 in neuroblastoma via inhibition of the ABCB1 efflux transporter.Sci Rep. 2017 Jun 8;7(1):3091. doi: 10.1038/s41598-017-03129-6. Sci Rep. 2017. PMID: 28596528 Free PMC article.
-
Analyzing the Blueprint: Exploring the Molecular Profile of Metastasis and Therapeutic Resistance.Int J Mol Sci. 2025 Jul 20;26(14):6954. doi: 10.3390/ijms26146954. Int J Mol Sci. 2025. PMID: 40725201 Free PMC article. Review.
-
New Evidence for P-gp-Mediated Export of Amyloid-β PEPTIDES in Molecular, Blood-Brain Barrier and Neuronal Models.Int J Mol Sci. 2020 Dec 29;22(1):246. doi: 10.3390/ijms22010246. Int J Mol Sci. 2020. PMID: 33383667 Free PMC article.
References
-
- Brodeur GM, Castleberry RP: Neuroblastoma: effect of genetic factors on prognosis and treatment. Cancer 1992, 70:1685-1694 - PubMed
-
- Brodeur G: Molecular basis for heterogeneity in human neuroblastoma. Eur J Cancer 1995, 31A:505-509 - PubMed
-
- Maris J, Matthay K: Molecular biology of neuroblastoma. J Clin Oncol 1999, 17:2264-2279 - PubMed
-
- Schwab M, Varmus HE, Bishop JM: Human N-myc gene contributes to neoplastic transformation of mammalian cell in culture. Nature 1985, 316:160-162 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

