Cytadherence-deficient mutants of Mycoplasma gallisepticum generated by transposon mutagenesis
- PMID: 12819064
- PMCID: PMC162017
- DOI: 10.1128/IAI.71.7.3812-3820.2003
Cytadherence-deficient mutants of Mycoplasma gallisepticum generated by transposon mutagenesis
Abstract
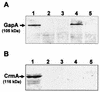
Cytadherence-related molecules of Mycoplasma gallisepticum strain R-low were identified by Tn4001 transposon mutagenesis with the hemadsorption (HA) assay as an indicator for cytadherence. Three Gm(r) HA-negative (HA(-)) colonies displaying a stable HA(-) phenotype through several successive generations in which gentamicin selection was maintained were isolated from four independent transformation experiments and characterized. Southern blot analysis showed that the transposon was inserted as a single copy within the genome of each of the HA(-) mutants, suggesting that the transposon insertion was directly responsible for their inability to attach to erythrocytes. Sequence analysis of the transposon insertion sites revealed that in two mutants, the transposon was inserted at two distinct sites within the gapA structural gene. In the third mutant, the insertion was mapped within the crmA gene, which is located immediately downstream of the gapA gene as part of the same operon. In vitro attachment experiments with the MRC-5 human lung fibroblast cell line showed that the cytadherence capabilities of the HA(-) mutants were less than 25% those of original strain R. Experimental infection of chickens, the natural host of M. gallisepticum, with each of the three mutants demonstrated significantly impaired colonization and host responses. These data demonstrate conclusively the role of both GapA and CrmA proteins in the adherence of M. gallisepticum to host cells in model systems and in vivo colonization. Furthermore, these results underscore the relevance of in vitro cytadherence model systems for studying the pathogenesis of natural infections in chickens.
Figures



Similar articles
-
GapA and CrmA coexpression is essential for Mycoplasma gallisepticum cytadherence and virulence.Infect Immun. 2002 Dec;70(12):6839-45. doi: 10.1128/IAI.70.12.6839-6845.2002. Infect Immun. 2002. PMID: 12438360 Free PMC article.
-
Isolation and characterization of transposon Tn4001-generated, cytadherence-deficient transformants of Mycoplasma pneumoniae and Mycoplasma genitalium.FEMS Immunol Med Microbiol. 1996 Oct;15(4):199-211. doi: 10.1111/j.1574-695X.1996.tb00086.x. FEMS Immunol Med Microbiol. 1996. PMID: 8908481
-
Role of the GapA and CrmA cytadhesins of Mycoplasma gallisepticum in promoting virulence and host colonization.Infect Immun. 2013 May;81(5):1618-24. doi: 10.1128/IAI.00112-13. Epub 2013 Mar 4. Infect Immun. 2013. PMID: 23460514 Free PMC article.
-
Haemagglutinins of pathogenic avian mycoplasmas.Avian Pathol. 2002 Dec;31(6):535-47. doi: 10.1080/0307945021000024526. Avian Pathol. 2002. PMID: 12593736 Review.
-
Mycoplasma pneumoniae cytadherence: unravelling the tie that binds.Mol Microbiol. 1996 Apr;20(2):247-53. doi: 10.1111/j.1365-2958.1996.tb02613.x. Mol Microbiol. 1996. PMID: 8733224 Review.
Cited by
-
Molecular cloning and characterization of a surface-localized adhesion protein in Mycoplasma bovis Hubei-1 strain.PLoS One. 2013 Jul 23;8(7):e69644. doi: 10.1371/journal.pone.0069644. Print 2013. PLoS One. 2013. PMID: 23936063 Free PMC article.
-
Immunologic Pathways in Protective versus Maladaptive Host Responses to Attenuated and Pathogenic Strains of Mycoplasma gallisepticum.Infect Immun. 2019 Feb 21;87(3):e00613-18. doi: 10.1128/IAI.00613-18. Print 2019 Mar. Infect Immun. 2019. PMID: 30559221 Free PMC article.
-
Comparative genomic analyses of attenuated strains of Mycoplasma gallisepticum.Infect Immun. 2010 Apr;78(4):1760-71. doi: 10.1128/IAI.01172-09. Epub 2010 Feb 1. Infect Immun. 2010. PMID: 20123709 Free PMC article.
-
Current status of vaccine research, development, and challenges of vaccines for Mycoplasma gallisepticum.Poult Sci. 2020 Sep;99(9):4195-4202. doi: 10.1016/j.psj.2020.06.014. Epub 2020 Jun 27. Poult Sci. 2020. PMID: 32867963 Free PMC article. Review.
-
Transcriptional responses of Mycoplasma gallisepticum strain R in association with eukaryotic cells.J Bacteriol. 2007 Aug;189(16):5803-7. doi: 10.1128/JB.00667-07. Epub 2007 Jun 8. J Bacteriol. 2007. PMID: 17557819 Free PMC article.
References
-
- Bradbury, J. M., O. M. Saed, C. A. Yavari, J. P. Dupiellet, and J. M. Bove. 1993. Mycoplasma imitans sp. nov. is related to Mycoplasma gallisepticum and found in birds. Int. J. Syst. Bacteriol. 43:721-728. - PubMed
-
- Byrne, M. E., D. A. Rouch, and R. A. Skurray. 1989. Nucleotide sequence analysis of IS256 from the Staphylococcus aureus gentamicin-tobramicin-kanamicin-resistance transposon Tn4001. Gene 81:361-367. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

