Construction and characterization of a Pseudomonas aeruginosa mucoid exopolysaccharide-alginate conjugate vaccine
- PMID: 12819072
- PMCID: PMC162014
- DOI: 10.1128/IAI.71.7.3875-3884.2003
Construction and characterization of a Pseudomonas aeruginosa mucoid exopolysaccharide-alginate conjugate vaccine
Abstract
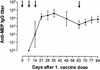
Deterioration of lung function in patients with cystic fibrosis (CF) is closely associated with chronic pulmonary infection with mucoid Pseudomonas aeruginosa. The mucoid exopolysaccharide (MEP) from P. aeruginosa has been shown to induce opsonic antibodies in mice that are protective against this chronic infection. MEP-specific opsonic antibodies are also commonly found in the sera of older CF patients lacking detectable P. aeruginosa infection. When used in a human vaccine trial, however, MEP only minimally induced opsonic antibodies. To evaluate whether conjugation of MEP to a carrier protein could improve its immunogenicity, we bound thiolated MEP to keyhole limpet hemocyanin (KLH) by using succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC) as a linker. In contrast to the native MEP polymer, the MEP-KLH conjugate vaccine induced high titers of MEP-specific immunoglobulin G (IgG) in C3H-HeN mice and in a rabbit. Sera from mice immunized with MEP-KLH conjugate, but not from animals immunized with comparable doses of native MEP, demonstrated opsonic killing activity. Vaccination with MEP-KLH conjugate induced opsonic antibodies broadly cross-reactive to heterologous mucoid strains of P. aeruginosa. Preexisting nonopsonic antibodies to MEP are found in normal human sera, including young CF patients, and their presence impedes the induction of opsonic antibodies. Induction of nonopsonic antibodies by either intraperitoneal injection of MEP or injection or feeding of the cross-reactive antigen, seaweed alginate, reduced the level of overall IgG elicited by follow-up immunization with the MEP-KLH conjugate. However, the opsonic activity was lower only in the sera of MEP-KLH conjugate-immunized mice with preexisting antibodies induced by MEP but not with antibodies induced by seaweed alginate. Immunization with MEP-KLH elicited a significant proportion of antibodies specific to epitopes involving O-acetate residues, and this subpopulation of antibodies mediated opsonic killing of mucoid P. aeruginosa in vitro. These results indicate that conjugation of MEP to KLH significantly enhances its immunogenicity and the elicitation of opsonic antibodies in mice and rabbits, that the conjugate induces opsonic antibodies in the presence of preexisting nonopsonic antibodies, and that opsonic antibodies to MEP are directed at epitopes that include acetate residues on the uronic acid polymer.
Figures



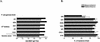
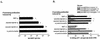

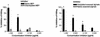
References
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
-
- Coleman, F. T., S. Mueschenborn, G. Meluleni, C. Ray, V. J. Carey, S. O. Vargas, C. L. Cannon, F. M. Ausubel, and G. B. Pier. 2003. Hypersusceptibility of cystic fibrosis mice to chronic Pseudomonas aeruginosa oropharyngeal colonization and lung infection. Proc. Natl. Acad. Sci. USA 100:1949-1954. - PMC - PubMed
-
- Demko, C. A., P. J. Byard, and P. B. Davis. 1995. Gender differences in cystic fibrosis: Pseudomonas aeruginosa infection. J. Clin. Epidemiol. 48:1041-1049. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

