Borrelia burgdorferi-induced tolerance as a model of persistence via immunosuppression
- PMID: 12819085
- PMCID: PMC162029
- DOI: 10.1128/IAI.71.7.3979-3987.2003
Borrelia burgdorferi-induced tolerance as a model of persistence via immunosuppression
Abstract
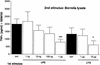
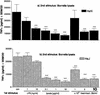
If left untreated, infection with Borrelia burgdorferi sensu lato may lead to chronic Lyme borreliosis. It is still unknown how this pathogen manages to persist in the host in the presence of competent immune cells. It was recently reported that Borrelia suppresses the host's immune response, thus perhaps preventing the elimination of the pathogen (I. Diterich, L. Härter, D. Hassler, A. Wendel, and T. Hartung, Infect. Immun. 69:687-694, 2001). Here, we further characterize Borrelia-induced immunomodulation in order to develop a model of this anergy. We observed that the different Borrelia preparations that we tested, i.e., live, heat-inactivated, and sonicated Borrelia, could desensitize human blood monocytes, as shown by attenuated cytokine release upon restimulation with any of the different preparations. Next, we investigated whether these Borrelia-specific stimuli render monocytes tolerant, i.e. hyporesponsive, towards another Toll-like receptor 2 (TLR2) agonist, such as lipoteichoic acid from gram-positive bacteria, or towards the TLR4 agonist lipopolysaccharide. Cross-tolerance towards all tested stimuli was induced. Furthermore, using primary bone marrow cells from TLR2-deficient mice and from mice with a nonfunctional TLR4 (strain C3H/HeJ), we demonstrated that the TLR2 was required for tolerance induction by Borrelia, and using neutralizing antibodies, we identified interleukin-10 as the key mediator involved. Although peripheral blood mononuclear cells tolerized by Borrelia exhibited reduced TLR2 and TLR4 mRNA levels, the expression of the respective proteins on monocytes was not decreased, ruling out the possibility that tolerance to Borrelia is attributed to a reduced TLR2 expression. In summary, we characterized tolerance induced by B. burgdorferi, describing a model of desensitization which might mirror the immunosuppression recently attributed to the persistence of Borrelia in immunocompetent hosts.
Figures





References
-
- Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285:736-739. - PubMed
-
- Barsig, J., D. S. Bundschuh, T. Hartung, A. Bauhofer, A. Sauer, and A. Wendel. 1996. Control of fecal peritoneal infection in mice by colony-stimulating factors. J. Infect. Dis. 174:790-799. - PubMed
-
- Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285:732-736. - PubMed
-
- Brooke, M. S. 1965. Conversion of immunological paralysis to immunity by endotoxin. Nature 206:635-636. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

