The Drosophila melanogaster toll pathway participates in resistance to infection by the gram-negative human pathogen Pseudomonas aeruginosa
- PMID: 12819096
- PMCID: PMC162001
- DOI: 10.1128/IAI.71.7.4059-4066.2003
The Drosophila melanogaster toll pathway participates in resistance to infection by the gram-negative human pathogen Pseudomonas aeruginosa
Abstract
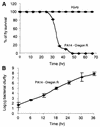
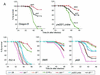
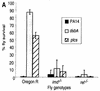
Pseudomonas aeruginosa is a gram-negative pathogen that infects immunocompromised and cystic fibrosis patients. The molecular basis of the host-P. aeruginosa interaction and the effect of specific P. aeruginosa virulence factors on various components of the innate immunity pathways are largely unknown. We examine interactions between P. aeruginosa virulence factors and components of innate immunity response in the Drosophila melanogaster model system to reveal the importance of the Toll signaling pathway in resistance to infection by the P. aeruginosa human isolate PA14. Using the two PA14-isogenic mutants plcS and dsbA, we show that Drosophila loss-of-function mutants of Spatzle, the extracellular ligand of Toll, and Dorsal and Dif, two NF-kappa B-like transcription factors, allow increased P. aeruginosa infectivity within fly tissues. In contrast, a constitutively active Toll mutant and a loss-of-function mutant of Cactus, an I kappa B-like factor that inhibits the Toll signaling, reduce infectivity. Our finding that Dorsal activity is required to restrict P. aeruginosa infectivity in Drosophila provides direct in vivo evidence for Dorsal function in adult fly immunity. Additionally, our results provide the basis for future studies into interactions between P. aeruginosa virulence factors and components of the Toll signaling pathway, which is functionally conserved between flies and humans.
Figures



References
-
- Anderson, K. V. 2000. Toll signaling pathways in the innate immune response. Curr. Opin. Immunol. 12:13-19. - PubMed
-
- Boman, H. G., I. Nilsson, and B. Rasmuson. 1972. Inducible antibacterial defense system in Drosophila. Nature 237:232-235. - PubMed
-
- Cox, D. R. 1972. Regression models and life tables. J. Royal Stat. Soc. Ser. B 34:187-220.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

