Effects of vitamin E supplementation during erythropoietin treatment of the anaemia of prematurity
- PMID: 12819167
- PMCID: PMC1721575
- DOI: 10.1136/fn.88.4.f324
Effects of vitamin E supplementation during erythropoietin treatment of the anaemia of prematurity
Abstract
Aims: To evaluate the effects of vitamin E supplementation on haemoglobin concentration and the requirement for transfusion in premature infants treated with erythropoietin and iron.
Methods: Randomised, double blind, placebo controlled trial. Thirty infants </=32 weeks gestation and </=1250 g birth weight, who were defined as stable based on minimal requirements for respiratory support and phlebotomy, and absence of major congenital anomalies were enrolled. All were treated with erythropoietin and iron, and were randomised to receive, in addition, either vitamin E 50 IU/day or placebo for eight weeks or until discharge, whichever came first.
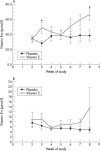
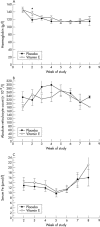
Results: Despite higher vitamin E (alpha-tocopherol) levels in the experimental group in weeks 3 (49.0 v 28.1 micro mol/l) and 8 (66.2 v 38.5 micro mol/l), there were no differences in haemoglobin, reticulocyte count, iron concentration, or transfusion requirement.
Conclusions: Oral vitamin E supplementation at 50 IU/day does not increase the response of preterm infants to erythropoietin and iron. Vitamin E obtained through standard nutrition may have been sufficient or higher doses may be required.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
