Analysis of Tat transactivation of human immunodeficiency virus transcription in vitro
- PMID: 1282057
- PMCID: PMC6057369
Analysis of Tat transactivation of human immunodeficiency virus transcription in vitro
Abstract
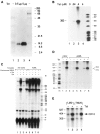
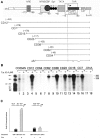
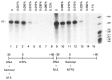
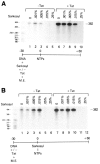
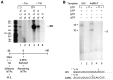
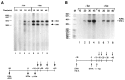
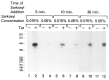
The HIV Tat protein is a potent transactivator of HIV transcription, increasing both RNA initiation and elongation. We now demonstrate that purified, full-length 86 amino acid Tat protein specifically transactivates the HIV LTR in vitro to a high level (25- to 60-fold). Tat transactivation was specifically blocked by anti-Tat serum, but not preimmune serum. Tat did not transactivate transcription from the control adenovirus major late promoter (AdMLP). HIV transcription was blocked at various functional steps during initiation and elongation complex formation. Similar to the control AdMLP, HIV basal initiation complex assembly was sensitive to the addition of 0.015% sarkosyl prior to the addition of nucleoside triphosphates. Resistance to 0.05% sarkosyl required the addition of G, C, and U, which constitute the first 13 bases of the HIV RNA transcript. The addition of Tat to the in vitro transcription relieved the 0.015% sarkosyl block. These Tat-induced complexes were sensitive to 0.05% sarkosyl, suggesting that transcriptional initiation had not occurred. Consistent with this hypothesis, the addition of G, C, and U to the Tat-induced transcription complexes allowed the rapid conversion to transcription initiation complexes. Tat also facilitated the formation of 0.015% sarkosyl-resistant complexes in a reconstituted transcription system containing partially purified transcription factors and polymerase II. Following the formation of stable initiation complexes, Tat increased the rate and efficiency of transcription elongation on the HIV but not the AdML template. Kinetic analysis of Tat transactivation suggests that approximately 30% of the Tat initiation complexes are converted to elongation complexes. We conclude that Tat, in addition to its demonstrated role in RNA elongation, facilitates transcription initiation in vitro.
Figures

Similar articles
-
HIV-1 Tat overcomes inefficient transcriptional elongation in vitro.J Mol Biol. 1993 Aug 5;232(3):732-46. doi: 10.1006/jmbi.1993.1427. J Mol Biol. 1993. PMID: 7689112
-
C-terminal domain phosphatase sensitivity of RNA polymerase II in early elongation complexes on the HIV-1 and adenovirus 2 major late templates.J Biol Chem. 2000 Oct 20;275(42):32430-7. doi: 10.1074/jbc.M005898200. J Biol Chem. 2000. PMID: 10938286
-
Distinct transcriptional pathways of TAR-dependent and TAR-independent human immunodeficiency virus type-1 transactivation by Tat.Virology. 1997 Aug 18;235(1):48-64. doi: 10.1006/viro.1997.8672. Virology. 1997. PMID: 9300036
-
Multiple modes of transcriptional regulation by the HIV-1 Tat transactivator.IUBMB Life. 2001 Mar;51(3):175-81. doi: 10.1080/152165401753544241. IUBMB Life. 2001. PMID: 11547919 Review.
-
Tackling Tat.J Mol Biol. 1999 Oct 22;293(2):235-54. doi: 10.1006/jmbi.1999.3060. J Mol Biol. 1999. PMID: 10550206 Review.
Cited by
-
Infection by Diverse HIV-1 Subtypes Leads to Different Elevations in HERV-K Transcriptional Levels in Human T Cell Lines.Front Microbiol. 2021 May 17;12:662573. doi: 10.3389/fmicb.2021.662573. eCollection 2021. Front Microbiol. 2021. PMID: 34079529 Free PMC article.
-
Transcription through the HIV-1 nucleosomes: effects of the PBAF complex in Tat activated transcription.Virology. 2010 Sep 30;405(2):322-33. doi: 10.1016/j.virol.2010.06.009. Virology. 2010. PMID: 20599239 Free PMC article.
-
Modulation of human immunodeficiency virus 1 replication by interferon regulatory factors.J Exp Med. 2002 May 20;195(10):1359-70. doi: 10.1084/jem.20010753. J Exp Med. 2002. PMID: 12021315 Free PMC article.
-
Effect of SWI/SNF chromatin remodeling complex on HIV-1 Tat activated transcription.Retrovirology. 2006 Aug 7;3:48. doi: 10.1186/1742-4690-3-48. Retrovirology. 2006. PMID: 16893449 Free PMC article.
-
Loss of G(1)/S checkpoint in human immunodeficiency virus type 1-infected cells is associated with a lack of cyclin-dependent kinase inhibitor p21/Waf1.J Virol. 2000 Jun;74(11):5040-52. doi: 10.1128/jvi.74.11.5040-5052.2000. J Virol. 2000. PMID: 10799578 Free PMC article.
References
-
- Abmayr S. M., Workman J. L., and Roeder R. G. (1988), Genes Dev 2, 542–553. - PubMed
-
- Arya S. K., Guo C., Josephs S. F., and Wong-Staal F. (1985), Science 229, 69–73. - PubMed
-
- Barre-Sinoussi T., Chermann J. C., Reye F., Nugeure M. T., Charmaret S., Gruest J., Dauquet C., Aixer-Blin C., Vezinet-Brun F., Rouzious C., Rozenbaum W., and Montagnier L. (1983), Science 220, 868–871. - PubMed
-
- Berkhout B., Gatignol A., Rabson A. B., and Jeang K.-T. (1990), Cell 62, 757–767. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
