Integrin alphavbeta3, requirement for VEGFR2-mediated activation of SAPK2/p38 and for Hsp90-dependent phosphorylation of focal adhesion kinase in endothelial cells activated by VEGF
- PMID: 12820653
- PMCID: PMC514852
- DOI: 10.1379/1466-1268(2003)8<37:ivrfva>2.0.co;2
Integrin alphavbeta3, requirement for VEGFR2-mediated activation of SAPK2/p38 and for Hsp90-dependent phosphorylation of focal adhesion kinase in endothelial cells activated by VEGF
Abstract
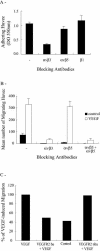
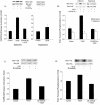
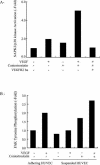
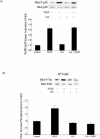
Endothelial cell migration, a key process in angiogenesis, requires the coordinated integration of motogenic signals elicited by the adhesion of endothelial cells to extracellular matrices and by angiogenic cytokines such as the vascular endothelial growth factor (VEGF). In this study, we found that addition of VEGF to human umbilical vein endothelial cells cultivated on vitronectin triggers a synergistic interaction between the VEGF receptor VEGFR2 and the clustered integrin receptor alphavbeta3. The interaction between VEGFR2 and alphavbeta3 is required for full phosphorylation of VEGFR2 and to drive the activation of motogenic pathways involving focal adhesion kinase (FAK) and stress-activated protein kinase-2/p38 (SAPK2/p38). The signal emanating from the VEGFR2 and alphavbeta3 interaction and leading to SAPK2/p38 activation proceeds directly from VEGFR2. The chaperone Hsp90 is found in a complex that coprecipitates with inactivated VEGFR2, and the association is increased by VEGF and decreased by geldanamycin, a specific inhibitor of Hsp90-mediated events. Geldanamycin also impairs the phosphorylation of FAK that results from the interaction between VEGFR2 and alphavbeta3, and this is accompanied by an inhibition of the recruitment of vinculin to VEGFR2. We conclude that a necessary cross talk should occur between VEGFR2 and the integrin alphavbeta3, to transduce the VEGF signals to SAPK2/p38 and FAK and that Hsp90 is instrumental in the building up of focal adhesions by allowing the phosphorylation of FAK and the recruitment of vinculin to VEGFR2.
Figures



Similar articles
-
Vascular endothelial growth factor (VEGF)-driven actin-based motility is mediated by VEGFR2 and requires concerted activation of stress-activated protein kinase 2 (SAPK2/p38) and geldanamycin-sensitive phosphorylation of focal adhesion kinase.J Biol Chem. 2000 Apr 7;275(14):10661-72. doi: 10.1074/jbc.275.14.10661. J Biol Chem. 2000. PMID: 10744763
-
Regulation of vascular endothelial growth factor receptor 2-mediated phosphorylation of focal adhesion kinase by heat shock protein 90 and Src kinase activities.J Biol Chem. 2004 Sep 10;279(37):39175-85. doi: 10.1074/jbc.M405493200. Epub 2004 Jul 6. J Biol Chem. 2004. PMID: 15247219
-
Phosphorylation of focal adhesion kinase (FAK) on Ser732 is induced by rho-dependent kinase and is essential for proline-rich tyrosine kinase-2-mediated phosphorylation of FAK on Tyr407 in response to vascular endothelial growth factor.Mol Biol Cell. 2006 Aug;17(8):3508-20. doi: 10.1091/mbc.e05-12-1158. Epub 2006 Jun 7. Mol Biol Cell. 2006. PMID: 16760434 Free PMC article.
-
Biochemical signals and biological responses elicited by the focal adhesion kinase.Biochim Biophys Acta. 2001 Jul 25;1540(1):1-21. doi: 10.1016/s0167-4889(01)00123-9. Biochim Biophys Acta. 2001. PMID: 11476890 Review.
-
Cooperation between integrin alphavbeta3 and VEGFR2 in angiogenesis.Angiogenesis. 2009;12(2):177-85. doi: 10.1007/s10456-009-9141-9. Epub 2009 Mar 8. Angiogenesis. 2009. PMID: 19267251 Free PMC article. Review.
Cited by
-
Signal transduction by vascular endothelial growth factor receptors.Cold Spring Harb Perspect Med. 2012 Jul;2(7):a006502. doi: 10.1101/cshperspect.a006502. Cold Spring Harb Perspect Med. 2012. PMID: 22762016 Free PMC article. Review.
-
Targeting HSP90 as a Novel Therapy for Cancer: Mechanistic Insights and Translational Relevance.Cells. 2022 Sep 6;11(18):2778. doi: 10.3390/cells11182778. Cells. 2022. PMID: 36139353 Free PMC article. Review.
-
Engineering a Chemically Defined Hydrogel Bioink for Direct Bioprinting of Microvasculature.Biomacromolecules. 2021 Feb 8;22(2):275-288. doi: 10.1021/acs.biomac.0c00947. Epub 2020 Dec 17. Biomacromolecules. 2021. PMID: 33332959 Free PMC article.
-
Calcium-dependent FAK/CREB/TNNC1 signalling mediates the effect of stromal MFAP5 on ovarian cancer metastatic potential.Nat Commun. 2014 Oct 3;5:5092. doi: 10.1038/ncomms6092. Nat Commun. 2014. PMID: 25277212 Free PMC article.
-
Endothelial Cell mTOR Complex-2 Regulates Sprouting Angiogenesis.PLoS One. 2015 Aug 21;10(8):e0135245. doi: 10.1371/journal.pone.0135245. eCollection 2015. PLoS One. 2015. PMID: 26295809 Free PMC article.
References
-
- Aplin AE, Howe A, Alahari SK, Juliano RL. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol Rev. 1998;50:197–263. - PubMed
-
- Borges E, Jan Y, Ruoslahti E. PDGF-receptor-(beta) and EGF-receptor-2 bind to the (beta)3 integrin through its extracellular domain. J Biol Chem. 2000;275:39867–39873. - PubMed
-
- Byzova TV, Goldman CK, Pampori N, Thomas KA, Bett A, Shattil SJ, Plow EF. A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol Cell. 2000;6:851–860. - PubMed
-
- Dedhar S, Williams B, Hannigan G. Integrin-linked kinase (ILK): a regulator of integrin and growth-factor signalling. Trends Cell Biol. 1999;9:319–323. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
