Multiple-dose safety and pharmacokinetics of oral garenoxacin in healthy subjects
- PMID: 12821477
- PMCID: PMC161848
- DOI: 10.1128/AAC.47.7.2256-2263.2003
Multiple-dose safety and pharmacokinetics of oral garenoxacin in healthy subjects
Abstract
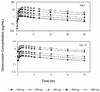
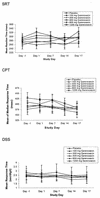
Garenoxacin (T-3811ME, BMS-284756) is a novel des-F(6) quinolone that has been shown to be effective in vitro against a wide range of clinically important pathogens, including gram-positive and gram-negative aerobes and anaerobes. This study was conducted to evaluate the safety and tolerability of multiple oral doses (100 to 1200 mg/day) of garenoxacin in healthy subjects and to determine its multiple-dose pharmacokinetics. Forty healthy male and female subjects (18 to 45 years of age) were enrolled in this randomized, double-blind, placebo-controlled, sequential, multiple- and ascending-dose study. Each subject received a once-daily oral dose of garenoxacin (100, 200, 400, 800, or 1200 mg) or a placebo for 14 days. Blood and urine samples were collected for measurements of garenoxacin by validated liquid chromatography with dual mass spectrometry, and plasma garenoxacin concentration-time data were analyzed by noncompartmental methods. The effects of garenoxacin on Helicobacter pylori, psychometric test performance, and electrocardiograms were assessed, as was drug safety. Over the 14 days of dosing, geometric mean peak concentrations of garenoxacin in plasma (C(max)) at the 100- and 1200-mg doses were within the ranges of 1.2 to 1.6 and 16.3 to 24 microg/ml, respectively. The corresponding values for the geometric mean area under the concentration-time curve over the dosing interval (AUC(tau)) for garenoxacin in plasma at the 100- and 1200-mg doses were within the ranges of 11.5 to 15.7 and 180 to 307 microg. h/ml, respectively. Increases in systemic exposure to garenoxacin in terms of AUC and C(max) were approximately dose proportional over the 100- to 400-mg dose range but demonstrated increases that were somewhat greater than the dose increments at the 800- and 1200-mg doses. Median values for the time to achieve C(max) were in the range of 1.13 to 2.50 h for all doses. The mean elimination half-life for garenoxacin in plasma appeared to be independent of dose and ranged from 13.3 to 17.8 h (day 14). Approximately 30 to 50% of an administered garenoxacin dose was excreted unchanged in the urine. At doses of 100 to 400 mg, steady-state concentrations of garenoxacin in plasma appeared to be attained by the fourth dose. Multiple oral doses of garenoxacin were well tolerated and did not demonstrate clinically significant effects on QT(c) or psychometric test results. Garenoxacin administered alone for 14 days at doses of >or=400 mg demonstrated activity against H. pylori. These results suggest that multiple once-daily oral doses of garenoxacin of up to 1200 mg are safe and well tolerated and that the pharmacokinetics of garenoxacin support once-daily administration.
Figures


Similar articles
-
Safety and clinical pharmacokinetics of nemonoxacin, a novel non-fluorinated quinolone, in healthy Chinese volunteers following single and multiple oral doses.Clin Drug Investig. 2012 Jul 1;32(7):475-86. doi: 10.2165/11632780-000000000-00000. Clin Drug Investig. 2012. PMID: 22650326 Clinical Trial.
-
Concentrations of the des-F(6)-quinolone garenoxacin in plasma and joint cartilage of immature rats.Arch Toxicol. 2004 Feb;78(2):61-7. doi: 10.1007/s00204-003-0514-3. Epub 2003 Dec 6. Arch Toxicol. 2004. PMID: 14661070
-
Phase I Clinical Study of ZYAN1, A Novel Prolyl-Hydroxylase (PHD) Inhibitor to Evaluate the Safety, Tolerability, and Pharmacokinetics Following Oral Administration in Healthy Volunteers.Clin Pharmacokinet. 2018 Jan;57(1):87-102. doi: 10.1007/s40262-017-0551-3. Clin Pharmacokinet. 2018. PMID: 28508936 Free PMC article. Clinical Trial.
-
Tiotropium bromide. A review of its use as maintenance therapy in patients with COPD.Treat Respir Med. 2004;3(4):247-68. doi: 10.2165/00151829-200403040-00005. Treat Respir Med. 2004. PMID: 15350163 Review.
-
Review of nemonoxacin with special focus on clinical development.Drug Des Devel Ther. 2014 Jul 5;8:765-74. doi: 10.2147/DDDT.S63581. eCollection 2014. Drug Des Devel Ther. 2014. PMID: 25045247 Free PMC article. Review.
Cited by
-
Multiple-dose safety, tolerability, and pharmacokinetics of oral nemonoxacin (TG-873870) in healthy volunteers.Antimicrob Agents Chemother. 2010 Jan;54(1):411-7. doi: 10.1128/AAC.00683-09. Epub 2009 Nov 2. Antimicrob Agents Chemother. 2010. PMID: 19884374 Free PMC article. Clinical Trial.
-
Interpretation of antibiotic concentration ratios measured in epithelial lining fluid.Antimicrob Agents Chemother. 2008 Jan;52(1):24-36. doi: 10.1128/AAC.00133-06. Epub 2007 Sep 10. Antimicrob Agents Chemother. 2008. PMID: 17846133 Free PMC article. Review. No abstract available.
-
Optimization Strategies Aimed to Increase the Efficacy of Helicobacter pylori Eradication Therapies with Quinolones.Molecules. 2020 Nov 2;25(21):5084. doi: 10.3390/molecules25215084. Molecules. 2020. PMID: 33147814 Free PMC article. Review.
-
Population pharmacokinetics and pharmacodynamics of garenoxacin in patients with community-acquired respiratory tract infections.Antimicrob Agents Chemother. 2004 Dec;48(12):4766-77. doi: 10.1128/AAC.48.12.4766-4777.2004. Antimicrob Agents Chemother. 2004. PMID: 15561855 Free PMC article.
-
Resistance of M. leprae to quinolones: a question of relativity?PLoS Negl Trop Dis. 2013 Nov 14;7(11):e2559. doi: 10.1371/journal.pntd.0002559. eCollection 2013 Nov. PLoS Negl Trop Dis. 2013. PMID: 24244784 Free PMC article.
References
-
- Applebaum, P. C. 1999. Quinolone activity against anaerobes. Drugs 58(Suppl. 2):60-64. - PubMed
-
- Ball, P., L. Mandell, Y. Niki, and G. Tillotson. 1999. Comparative tolerability of the newer floroquinolone antibacterials. Drug Saf. 21:407-421. - PubMed
-
- Bazett, H. C. 1920. An analysis of the time relations of electrocardiograms. Heart 7:353-370.
-
- Bertino, J., Jr., and D. Fish. 2000. The safety profile of the fluoroquinolones. Clin. Ther. 22:798-817. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

