Phot1 and phot2 mediate blue light-induced transient increases in cytosolic Ca2+ differently in Arabidopsis leaves
- PMID: 12821778
- PMCID: PMC166272
- DOI: 10.1073/pnas.1336802100
Phot1 and phot2 mediate blue light-induced transient increases in cytosolic Ca2+ differently in Arabidopsis leaves
Abstract
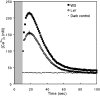
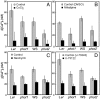
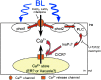
Phototropins (phot1 and phot2) are blue light (BL) receptors that mediate phototropism, chloroplast movements, and stomatal opening in Arabidopsis thaliana. Physiological studies have suggested that Ca2+ in the cytoplasm plays a pivotal role in these BL-induced responses. A phot1-mediated increase in cytosolic Ca2+ was reported in deetiolated seedlings of A. thaliana; however, the contribution of phot2 remains unknown. We examined a BL-induced transient increase in cytosolic free Ca2+ in leaves of transgenic A. thaliana of WT plants, phot1 and phot2 mutants, and phot1 phot2 double mutants expressing the Ca2+-sensitive luminescent protein aequorin. phot1 and phot2 had different photosensitivities: phot1 increased cytosolic Ca2+ at lower fluence rates (0.1-50 micromol x m-2 x s-1) and phot2 increased it at higher fluence rates (1-250 micromol x m-2 x s-1). By using Ca2+ channel blockers, Ca2+ chelating agents, and inhibitors of phospholipase C, we further demonstrated that both phot1 and phot2 could induce Ca2+ influx from the apoplast through the Ca2+ channel in the plasma membrane, whereas phot2 alone induced phospholipase C-mediated phosphoinositide signaling, which might result in Ca2+ release from internal Ca2+ stores. These results suggest that phot1 and phot2 mediate the BL-induced increase in cytosolic free Ca2+ differently.
Figures


Similar articles
-
Phototropins function in high-intensity blue light-induced hypocotyl phototropism in Arabidopsis by altering cytosolic calcium.Plant Physiol. 2013 Jul;162(3):1539-51. doi: 10.1104/pp.113.216556. Epub 2013 May 14. Plant Physiol. 2013. PMID: 23674105 Free PMC article.
-
Arabidopsis phot1 and phot2 phosphorylate BLUS1 kinase with different efficiencies in stomatal opening.J Plant Res. 2016 Mar;129(2):167-74. doi: 10.1007/s10265-015-0780-1. Epub 2016 Jan 16. J Plant Res. 2016. PMID: 26780063
-
Functional characterization of blue-light-induced responses and PHOTOTROPIN 1 gene in Welwitschia mirabilis.J Plant Res. 2016 Mar;129(2):175-87. doi: 10.1007/s10265-016-0790-7. Epub 2016 Feb 8. J Plant Res. 2016. PMID: 26858202
-
Phototropins and blue light-dependent calcium signaling in higher plants.Photochem Photobiol. 2007 Jan-Feb;83(1):102-11. doi: 10.1562/2006-03-08-IR-837. Photochem Photobiol. 2007. PMID: 16906793 Review.
-
Phototropins 1 and 2: versatile plant blue-light receptors.Trends Plant Sci. 2002 May;7(5):204-10. doi: 10.1016/s1360-1385(02)02245-8. Trends Plant Sci. 2002. PMID: 11992825 Review.
Cited by
-
Screening for Arabidopsis mutants with altered Ca2+ signal response using aequorin-based Ca2+ reporter system.STAR Protoc. 2021 May 23;2(2):100558. doi: 10.1016/j.xpro.2021.100558. eCollection 2021 Jun 18. STAR Protoc. 2021. PMID: 34041505 Free PMC article.
-
Link between Lipid Second Messengers and Osmotic Stress in Plants.Int J Mol Sci. 2021 Mar 6;22(5):2658. doi: 10.3390/ijms22052658. Int J Mol Sci. 2021. PMID: 33800808 Free PMC article. Review.
-
A plant-specific protein essential for blue-light-induced chloroplast movements.Plant Physiol. 2005 Sep;139(1):101-14. doi: 10.1104/pp.105.061887. Epub 2005 Aug 19. Plant Physiol. 2005. PMID: 16113226 Free PMC article.
-
In vivo reorganization of the actin cytoskeleton in leaves of Nicotiana tabacum L. transformed with plastin-GFP. Correlation with light-activated chloroplast responses.BMC Plant Biol. 2009 May 29;9:64. doi: 10.1186/1471-2229-9-64. BMC Plant Biol. 2009. PMID: 19480655 Free PMC article.
-
Temperature-dependent signal transmission in chloroplast accumulation response.J Plant Res. 2017 Jul;130(4):779-789. doi: 10.1007/s10265-017-0938-0. Epub 2017 Apr 18. J Plant Res. 2017. PMID: 28421371
References
-
- Briggs, W. R. & Christie, J. M. (2002) Trends Plant Sci. 7 204-210. - PubMed
-
- Huala, E., Oeller, P. W., Liscum, E., Han, I. S., Larsen, E. & Briggs, W. R. (1997) Science 278 2120-2123. - PubMed
-
- Kagawa, T., Sakai, T., Suetsugu, N., Oikawa, K., Ishiguro, S., Kato, T., Tabata, S., Okada, K. & Wada M. (2001) Science 291 2138-2141. - PubMed
-
- Christie, J. M., Reymond, P., Powell, G. K., Bernasconi, P., Raibekas, A. A., Liscum, E. & Briggs, W. R. (1998) Science 282 1698-1701. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

