Mdm2-dependent ubiquitination and degradation of the insulin-like growth factor 1 receptor
- PMID: 12821780
- PMCID: PMC166214
- DOI: 10.1073/pnas.1431613100
Mdm2-dependent ubiquitination and degradation of the insulin-like growth factor 1 receptor
Abstract
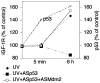
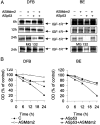
Recently, p53 was demonstrated to affect the expression of the insulin-like growth factor 1 receptor (IGF-1R), a receptor tyrosine kinase that plays a crucial role in growth and survival of cancer cells. However, the underlying mechanisms for interaction between p53 and IGF-1R are still not fully understood. One of the challenging questions remaining to be answered is why the wild-type p53, which per se represses the transcription of the IGF-1R gene, in overexpressed form is necessary for a high IGF-1R expression. In this study, we show that inhibition of p53 causes ubiquitination and down-regulation, through increased degradation, of the IGF-1R in human malignant melanoma cells. This effect, which was independent of the p53 status (i.e., wild type or mutated), was prevented if Mdm2 was coinhibited. Similar results were obtained in UV-irradiated human melanocytes (harboring wild-type p53), in which level of the IGF-1R increased after up-regulation of p53. Interestingly, the basal ubiquitination of the IGF-1R in untreated cells also depended on Mdm2. We could prove that Mdm2 physically associates with IGF-1R and that Mdm2 causes IGF-1R ubiquitination in an in vitro assay. Taken together our data provide evidence that Mdm2 serves as a ligase in ubiquitination of the IGF-1R and thereby causes its degradation by the proteasome system. Consequently, by sequestering Mdm2 in the cell nuclei, the level of p53 may indirectly influence the expression of IGF-1R. This role of Mdm2 and p53 represents an unexpected mechanism for the regulation of IGF-1R and cell growth.
Figures



References
-
- Baserga, R. (1995) Cancer Res. 55 249-252. - PubMed
-
- Baserga, R., Resnicoff, M. & Dews, M. (1997) Endocrine 7 99-102. - PubMed
-
- Werner, H. & Le Roith, D. (1997) Crit. Rev. Oncog. 8 71-92. - PubMed
-
- Buckbinder, L., Talbott, R., Velasco-Miguel, S., Takenaka, I., Faha, B., Seizinger, B. R. & Kley, N. (1995) Nature 377 646-649. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

