Interferon alpha therapy in patients with chronic type C hepatitis: changes of serum ALT, anti-HCV & HCV-RNA
- PMID: 1282364
- PMCID: PMC4532103
- DOI: 10.3904/kjim.1992.7.1.13
Interferon alpha therapy in patients with chronic type C hepatitis: changes of serum ALT, anti-HCV & HCV-RNA
Abstract
Background: After the discovery of type C hepatitis virus, the studies on this virus are extensively progressing. The treatment of this viral infection is also widely progressing. Among many agents, recombinant interferon alpha therapy is generally accepted as an effective single agent. To evaluate the efficacy of interferon and to observe the changes of serum aminotransferase (ALT), antibody to hepatitis C virus (anti-HCV) and HCV ribonucleic acid (HCV-RNA), we treated 10 patients with chronic type C hepatitis for 6 months.
Methods: Patients were randomly divided into 2 groups: 5 patients in group A received interferon and the other 5 in group B received no therapy. Interferon was administered at a dose of 3 million units (MU) daily for the first month and thrice weekly for the following 5 months, and followed up for 2 years.
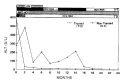
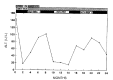
Results: In group A, serum ALT returned to normal in 4: 3, starting at the first month and one at the 3rd month of therapy and maintained normal throughout the follow-up period. In contrast, serum ALT level persistently fluctuated in 4 patients in group B. In one patient, serum ALT returned to normal one and a half years later. Regardless of therapy, serum anti-HCV titer remained unchanged in all patients. However, HCV-RNA, using polymerized chain reaction (PCR), became undetectable in all responded patients and in one untreated patient whose serum ALT returned to normal spontaneously.
Conclusion: This study suggested that interferon alpha therapy in patients with chronic type C hepatitis may be clinically effective. Our study also indicated that the detection of HCV-RNA by PCR is useful to predict the prognosis of chronic type C hepatitis.
Figures
Similar articles
-
Long-term carriage of hepatitis C virus with normal aminotransferase after interferon treatment in patients with chronic hepatitis C.J Med Virol. 1993 Sep;41(1):65-70. doi: 10.1002/jmv.1890410114. J Med Virol. 1993. PMID: 8228941
-
Serum HCV RNA levels in patients with chronic hepatitis C given a second course of interferon alpha-2b treatment after relapse following initial treatment.Scand J Infect Dis. 1993;25(1):25-30. Scand J Infect Dis. 1993. PMID: 8384732
-
Changes of serum hepatitis C virus levels in patients with chronic hepatitis C treated with two courses of interferon administration.Tohoku J Exp Med. 1994 Aug;173(4):361-9. doi: 10.1620/tjem.173.361. Tohoku J Exp Med. 1994. PMID: 7529950
-
Therapy of hepatitis C: patients with normal aminotransferase levels.Hepatology. 1997 Sep;26(3 Suppl 1):133S-136S. doi: 10.1002/hep.510260723. Hepatology. 1997. PMID: 9305678 Review.
-
Treatment of hepatitis C patients with normal aminotransferases levels.Clin Liver Dis. 1999 Nov;3(4):843-53. doi: 10.1016/s1089-3261(05)70242-7. Clin Liver Dis. 1999. PMID: 11291254 Review.
Cited by
-
Interferon for interferon nonresponding and relapsing patients with chronic hepatitis C.Cochrane Database Syst Rev. 2013 Jan 31;2013(1):CD003617. doi: 10.1002/14651858.CD003617.pub2. Cochrane Database Syst Rev. 2013. PMID: 23440791 Free PMC article.
References
-
- Choo QL, Kuo G, Weimer AJ, et al. Isolation of cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359. - PubMed
-
- Kuo G, Choo QL, Alter HJ, et al. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989;244:362. - PubMed
-
- Alter MJ. Hepatitis C and miles to go before we sleep. N Engl J Med. 1989;321:1538. - PubMed
-
- Govindarajan S, McHutchison JG, Vallinluck B, Redeker AG. Prevalence of anti-HCV in patients with hepato cellular carcinoma (Abstract) 1990;12(Part 2):878.
-
- Liang TJ, Jeffers LJ, Reddy RK, Wands JR, Schiff ER. HBV related and HCV infection in HBsAg negative patients with hepatocellular carcinoma (Abstract) Hepatology. 12(Part 2):881.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials