Activation and block of mouse muscle-type nicotinic receptors by tetraethylammonium
- PMID: 12824448
- PMCID: PMC2343137
- DOI: 10.1113/jphysiol.2003.043885
Activation and block of mouse muscle-type nicotinic receptors by tetraethylammonium
Abstract
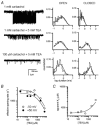
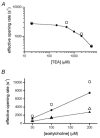
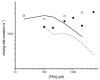
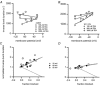
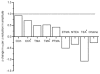
We have studied the activation and inhibition of the mouse muscle adult-type nicotinic acetylcholine receptor by tetraethylammonium (TEA) and related quaternary ammonium derivatives. The data show that TEA is a weak agonist of the nicotinic receptor. No single-channel clusters were observed at concentrations as high as 5 mM TEA or in the presence of a mutation which selectively increases the efficacy of the receptor. When coapplied with 1 mM carbamylcholine (CCh), TEA decreased the effective opening rate demonstrating that it acts as a competitive antagonist of CCh-mediated activation. Kinetic analysis of currents elicited by CCh and TEA allowed an estimate of receptor affinity for TEA of about 1 mM, while an upper limit of 10 s-1 could be set for the wild-type channel-opening rate constant for receptors activated by TEA alone. At millimolar concentrations, TEA inhibited nicotinic receptor currents by depressing the single-channel amplitude. The effect had an IC50 of 2-3 mM, depending on the conditions of the experiment, and resembled a standard open-channel block. However, the decrease in channel amplitudes was not accompanied by an increase in the mean burst duration, indicating that a linear open-channel blocking mechanism is not applicable. Upon studying block by other nicotinic receptor ligands it was found that block by CCh, tetramethylammonium and phenyltrimethylammonium can be accounted for by the sequential blocking mechanism while block in the presence of methyltriethylammonium, ethyltrimethylammonium or choline was inconsistent with such a mechanism. A mechanism in which receptors blocked by TEA can close would account for the experimental findings.
Figures
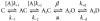

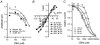

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

