Calmodulin kinase modulates Ca2+ release in mouse skeletal muscle
- PMID: 12824452
- PMCID: PMC2343155
- DOI: 10.1113/jphysiol.2003.042002
Calmodulin kinase modulates Ca2+ release in mouse skeletal muscle
Abstract
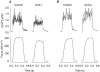
Activation of the contractile machinery in skeletal muscle is initiated by the action-potential-induced release of Ca2+ from the sarcoplasmic reticulum (SR). Several proteins involved in SR Ca2+ release are affected by calmodulin kinase II (CaMKII)-induced phosphorylation in vitro, but the effect in the intact cell remains uncertain and is the focus of the present study. CaMKII inhibitory peptide or inactive control peptide was injected into single isolated fast-twitch fibres of mouse flexor digitorum brevis muscles, and the effect on free myoplasmic [Ca2+] ([Ca2+]i) and force during different patterns of stimulation was measured. Injection of the inactive control peptide had no effect on any of the parameters measured. Conversely, injection of CaMKII inhibitory peptide decreased tetanic [Ca2+]i by ~25 %, but had no significant effect on the rate of SR Ca2+ uptake or the force-[Ca2+]i relationship. Repeated tetanic stimulation resulted in increased tetanic [Ca2+]i, and this increase was smaller after CaMKII inhibition. In conclusion, CaMKII-induced phosphorylation facilitates SR Ca2+ release in the basal state and during repeated contractions, providing a positive feedback between [Ca2+]i and SR Ca2+ release.
Figures
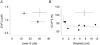


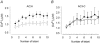
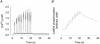

References
-
- Allen DG, Duty S, Westerblad H. Metabolic changes in muscle during exercise; their effect on muscle function. Proc Aust Physiol Soc. 1993;24:65–75.
-
- Anderson ME. Ca2+-dependent regulation of cardiac L-type Ca2+ channels: is a unifying mechanism at hand? J Mol Cell Cardiol. 2001;33:639–650. - PubMed
-
- Balog EM, Fruen BR, Kane PK, Louis CF. Mechanisms of Pi regulation of the skeletal muscle SR Ca2+ release channel. Am J Physiol. 2000;278:C601–611. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

