Bone marrow stem cells contribute to repair of the ischemically injured renal tubule
- PMID: 12824456
- PMCID: PMC162291
- DOI: 10.1172/JCI17856
Bone marrow stem cells contribute to repair of the ischemically injured renal tubule
Abstract
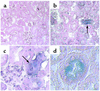
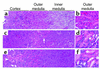
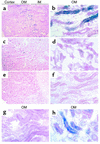
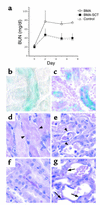
The paradigm for recovery of the renal tubule from acute tubular necrosis is that surviving cells from the areas bordering the injury must migrate into the regions of tubular denudation and proliferate to re-establish the normal tubular epithelium. However, therapies aimed at stimulating these events have failed to alter the course of acute renal failure in human trials. In the present study, we demonstrate that Lin-Sca-1+ cells from the adult mouse bone marrow are mobilized into the circulation by transient renal ischemia and home specifically to injured regions of the renal tubule. There they differentiate into renal tubular epithelial cells and appear to constitute the majority of the cells present in the previously necrotic tubules. Loss of stem cells following bone marrow ablation results in a greater rise in blood urea nitrogen after renal ischemia, while stem cell infusion after bone marrow ablation reverses this effect. Thus, therapies aimed at enhancing the mobilization, propagation, and/or delivery of bone marrow stem cells to the kidney hold potential as entirely new approaches for the treatment of acute tubular necrosis.
Figures


References
-
- Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N. Engl. J. Med. 1996;334:1448–1460. - PubMed
-
- Witzgall R, Brown D, Schwarz C, Bonventre JV. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J. Clin. Invest. 1994;93:2175–2188. - PMC - PubMed
-
- van Why SK, et al. Thresholds for cellular disruption and activation of the stress response in renal epithelia. Am. J. Physiol. 1999;277:F227–F234. - PubMed
-
- Al-Awqati Q, Oliver JA. Stem cells in the kidney. Kidney Int. 2002;61:387–395. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

