Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway
- PMID: 12824464
- PMCID: PMC166260
- DOI: 10.1073/pnas.1432609100
Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway
Abstract
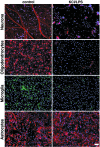
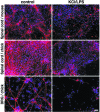
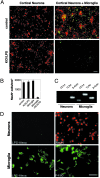
Innate immunity is an evolutionarily ancient system that provides organisms with immediately available defense mechanisms through recognition of pathogen-associated molecular patterns. We show that in the CNS, specific activation of innate immunity through a Toll-like receptor 4 (TLR4)-dependent pathway leads to neurodegeneration. We identify microglia as the major lipopolysaccharide (LPS)-responsive cell in the CNS. TLR4 activation leads to extensive neuronal death in vitro that depends on the presence of microglia. LPS leads to dramatic neuronal loss in cultures prepared from wild-type mice but does not induce neuronal injury in CNS cultures derived from tlr4 mutant mice. In an in vivo model of neurodegeneration, stimulating the innate immune response with LPS converts a subthreshold hypoxic-ischemic insult from no discernable neuronal injury to severe axonal and neuronal loss. In contrast, animals bearing a loss-of-function mutation in the tlr4 gene are resistant to neuronal injury in the same model. The present study demonstrates a mechanistic link among innate immunity, TLRs, and neurodegeneration.
Figures


References
-
- Kreutzberg, G. W. (1996) Trends Neurosci. 19 312-318. - PubMed
-
- Dickson, D. W., Lee, S. C., Mattiace, L. A., Yen, S. H. & Brosnan, C. (1993) Glia 7 75-83. - PubMed
-
- McGeer, P. L., Itagaki, S., Boyes, B. E. & McGeer, E. G. (1988) Neurology 38 1285-1291. - PubMed
-
- Trapp, B. D., Bo, L., Mork, S. & Chang, A. (1999) J. Neuroimmunol. 98 49-56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

