Regulatory CD8+ T cells fine-tune the myelin basic protein-reactive T cell receptor V beta repertoire during experimental autoimmune encephalomyelitis
- PMID: 12824465
- PMCID: PMC166237
- DOI: 10.1073/pnas.1432871100
Regulatory CD8+ T cells fine-tune the myelin basic protein-reactive T cell receptor V beta repertoire during experimental autoimmune encephalomyelitis
Abstract
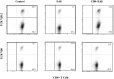
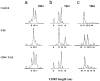
A significant number of self-reactive T cell clones escape thymic negative selection and are released into the periphery, where some are potentially pathogenic. The clonal expansion of self-reactive T cells is known to be limited during initial antigen encounter by apoptotic or anergic mechanisms, regulatory CD4+ T cells, and cytokines. Here we report that superimposed on these mechanisms, during the evolution of autoimmunity in experimental autoimmune encephalomyelitis (EAE), CD8+ T cells are induced, which fine-tune the peripheral self-reactive T cell receptor (TCR) repertoire. We assayed the myelin basic protein-reactive TCR repertoire in naive, EAE-recovered mice as well as EAE-recovered mice depleted of CD8+ T cells by TCRV beta surface expression, complementarity-determining region 3 length distribution, and complementarity-determining region 3 sequencing analysis. In EAE-recovered mice, certain myelin basic protein-reactive CD4+V beta 8.2+ clones are significantly decreased and this decrease is not observed if CD8+ T cells were depleted from these mice. The clones that persist in CD8+ T cell-intact mice are highly diverse in contrast to the clones expanded in CD8+ T cell-depleted mice, which are dominated by the significant outgrowth of a few clones. Importantly, the T cell clones that expand in the absence of CD8+ T cell control are enriched in potentially pathogenic self-reactive T cell clones capable of inducing EAE in vivo.
Figures


References
-
- Harrington, C. J., Paez, A., Hunkapiller, T., Mannikko, V., Brabb, T., Ahearn, M., Beeson, C. & Goverman, J. (1998) Immunity 8 571-580. - PubMed
-
- Goldrath, A. W. & Bevan, M. J. (1999) Nature 402 255-262. - PubMed
-
- Bouneaud, C., Kourilsky, P. & Bousso, P. (2000) Immunity 13 829-840. - PubMed
-
- Kuchroo, V. K., Anderson, A. C., Waldner, H., Munder, M., Bettelli, E. & Nicholson, L. B. (2002) Annu. Rev. Immunol. 20 101-123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

