Crystal structures of fusion proteins with large-affinity tags
- PMID: 12824478
- PMCID: PMC2323919
- DOI: 10.1110/ps.0243403
Crystal structures of fusion proteins with large-affinity tags
Abstract
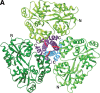
The fusion of a protein of interest to a large-affinity tag, such as the maltose-binding protein (MBP), thioredoxin (TRX), or glutathione-S-transferase (GST), can be advantageous in terms of increased expression, enhanced solubility, protection from proteolysis, improved folding, and protein purification via affinity chromatography. Unfortunately, crystal growth is hindered by the conformational heterogeneity induced by the fusion tag, requiring that the tag is removed by a potentially problematic cleavage step. The first three crystal structures of fusion proteins with large-affinity tags have been reported recently. All three structures used a novel strategy to rigidly fuse the protein of interest to MBP via a short three- to five-amino acid spacer. This strategy has the potential to aid structure determination of proteins that present particular experimental challenges and are not conducive to more conventional crystallization strategies (e.g., membrane proteins). Structural genomics initiatives may also benefit from this approach as a way to crystallize problematic proteins of significant interest.
Figures




References
-
- Abramson, J. and Iwata, S. 1999. Crystallization of membrane proteins. In Protein crystallization: Techniques, strategies, and tips: A laboratory manual (ed. T.M. Bergfors), pp. 199–210. International University Line, La Jolla, CA.
-
- Baneyx, F. 1999. Recombinant protein expression in Escherichia coli. Curr. Opin. Biotechnol. 10 411–421. - PubMed
-
- Beckwith, J. 2000. The all purpose gene fusion. Methods Enzymol. 326 3–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

