Understanding the function-structure and function-mutation relationships of p53 tumor suppressor protein by high-resolution missense mutation analysis
- PMID: 12826609
- PMCID: PMC166245
- DOI: 10.1073/pnas.1431692100
Understanding the function-structure and function-mutation relationships of p53 tumor suppressor protein by high-resolution missense mutation analysis
Abstract
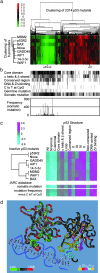
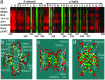
Inactivation of the tumor suppressor p53 by missense mutations is the most frequent genetic alteration in human cancers. The common missense mutations in the TP53 gene disrupt the ability of p53 to bind to DNA and consequently to transactivate downstream genes. However, it is still not fully understood how a large number of the remaining mutations affect p53 structure and function. Here, we used a comprehensive site-directed mutagenesis technique and a yeast-based functional assay to construct, express, and evaluate 2,314 p53 mutants representing all possible amino acid substitutions caused by a point mutation throughout the protein (5.9 substitutions per residue), and correlated p53 function with structure- and tumor-derived mutations. This high-resolution mutation analysis allows evaluation of previous predictions and hypotheses through interrelation of function, structure and mutation.
Figures


References
-
- el-Deiry, W. S., Kern, S. E., Pietenpol, J. A., Kinzler, K. W. & Vogelstein, B. (1992) Nat. Genet. 1 45-49. - PubMed
-
- el-Deiry, W. S. (1998) Semin. Cancer Biol. 8 345-357. - PubMed
-
- Cho, Y., Gorina, S., Jeffrey, P. D. & Pavletich, N. P. (1994) Science 265 346-355. - PubMed
-
- Clore, G. M., Omichinski, J. G., Sakaguchi, K., Zambrano, N., Sakamoto, H., Appella, E. & Gronenborn, A. M. (1994) Science 265 386-391. - PubMed
-
- Jeffrey, P. D., Gorina, S. & Pavletich, N. P. (1995) Science 267 1498-1502. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

