Mechanisms of selectivity in channels and enzymes studied with interactive molecular dynamics
- PMID: 12829462
- PMCID: PMC1303063
- DOI: 10.1016/S0006-3495(03)74452-X
Mechanisms of selectivity in channels and enzymes studied with interactive molecular dynamics
Abstract
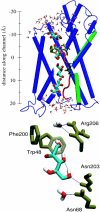
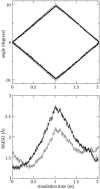
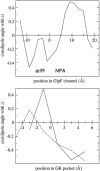
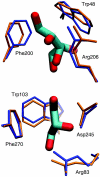
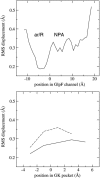
Interactive molecular dynamics, a new modeling tool for rapid investigation of the physical mechanisms of biological processes at the atomic level, is applied to study selectivity and regulation of the membrane channel protein GlpF and the enzyme glycerol kinase. These proteins facilitate the first two steps of Escherichia coli glycerol metabolism. Despite their different function and architecture the proteins are found to employ common mechanisms for substrate selectivity: an induced geometrical fit by structurally homologous binding sites and an induced rapid dipole moment reversal. Competition for hydrogen bonding sites with water in both proteins is critical for substrate motion. In glycerol kinase, it is shown that the proposed domain motion prevents competition with water, in turn regulating the binding of glycerol.
Figures
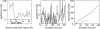

References
-
- Ai, Z., and T. Fröhlich. 1998. Molecular dynamics simulation in virtual environments. Comp. Graph. Forum. 17:C267–C273.
-
- Borgnia, M., S. Nielsen, A. Engel, and P. Agre. 1999. Cellular and molecular biology of the aquaporin water channels. Annu. Rev. Biochem. 68:425–458. - PubMed
-
- Bystrom, C. E., D. W. Pettigrew, B. P. Branchaud, P. O'Brien, and S. J. Remington. 1999. Crystal structures of Escherichia coli glycerol kinase variant S58→W in complex with nonhydrolyzable ATP analogues reveal a putative active conformation of the enzyme as a result of domain motion. Biochemistry. 38:3508–3518. - PubMed
-
- de Groot, B. L., A. Engel, and H. Grubmüller. 2001. A refined structure of human aquaporin-1. FEBS Lett. 504:206–211. - PubMed
-
- de Groot, B. L., and H. Grubmüller. 2001. Water permeation across biological membranes: mechanism and dynamics of aquaporin-1 and GlpF. Science. 294:2353–2357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

