Posttransition state desolvation of the hydrophobic core of the src-SH3 protein domain
- PMID: 12829464
- PMCID: PMC1303065
- DOI: 10.1016/S0006-3495(03)74454-3
Posttransition state desolvation of the hydrophobic core of the src-SH3 protein domain
Abstract
The folding thermodynamics of the src-SH3 protein domain were characterized under refolding conditions through biased fully atomic molecular dynamics simulations with explicit solvent. The calculated free energy surfaces along several reaction coordinates revealed two barriers. The first, larger barrier was identified as the transition state barrier for folding, associated with the formation of the first hydrophobic sheet of the protein. phi values calculated from structures residing at the transition state barrier agree well with experimental phi values. The microscopic information obtained from our simulations allowed us to unambiguously assign intermediate phi values as the result of multiple folding pathways. The second, smaller barrier occurs later in the folding process and is associated with the cooperative expulsion of water molecules between the hydrophobic sheets of the protein. This posttransition state desolvation barrier cannot be observed through traditional folding experiments, but is found to be critical to the correct packing of the hydrophobic core in the final stages of folding. Hydrogen exchange and NMR experiments are suggested to probe this barrier.
Figures
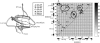





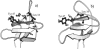
References
-
- Borreguero, J. M., N. V. Dokholyan, S. V. Buldyrev, E. I. Shakhnovich, and H. E. Stanley. 2002. Thermodynamics and folding kinetics analysis of the SH3 domain form discrete molecular dynamics. J. Mol. Biol. 318:863–876. - PubMed
-
- Brooks, B. R., R. E. Bruccoleri, B. D. Olafson, D. J. States, D. J. Swaminathan, and M. Karplus. 1982. CHARMM: a program for macromolecular energy, minimization, and dynamics calculations. J. Comput. Chem. 4:187–217.
-
- Bryngelson, J. D., J. N. Onuchic, and P. G. Wolynes. 1995. Funnels, pathways and the energy landscape of protein folding: a synthesis. Proteins. 21:167–195. - PubMed
-
- Bryngelson, J. D., and P. G. Wolynes. 1990. A simple statistical field theory of heteropolymer collapse with application to protein folding. Biopolymers. 30:177–188.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

