Concentration effect on the aggregation of a self-assembling oligopeptide
- PMID: 12829508
- PMCID: PMC1303109
- DOI: 10.1016/S0006-3495(03)74498-1
Concentration effect on the aggregation of a self-assembling oligopeptide
Abstract
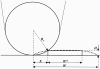
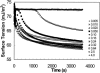
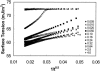
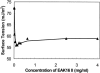
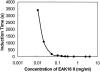
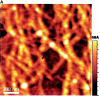
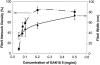
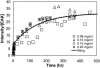
Concentration is a key parameter in controlling the aggregation of self-assembling oligopeptides. By investigating the concentration effects, an aggregation mechanism of EAK16-II is proposed. Depending on the critical aggregation concentration (CAC) of EAK16-II, the oligopeptide aggregates into protofibrils through seeding and/or a nucleation process. Protofibrils then associate with each other to form fibrils. The CAC was found to be approximately 0.1 mg/ml by surface tension measurements. The nanostructures of aggregates were imaged and analyzed by atomic force microscopy. Globular and fibrillar aggregates were observed, and their dimensions were further quantified. To ensure that the aggregates were formed in bulk solution, light scattering (LS) measurements were conducted to monitor the fibril formation with time. The LS profile showed two different rates of aggregation depending on whether the peptide concentration was above or below the CAC. At high concentrations, the LS intensity increased strongly at early times. At low concentrations, the LS intensity increased only slightly. Our study provides information about the nature of the oligopeptide self-assembly, which is important to the understanding of the fibrillogenesis occurring in conformational diseases and to many biomedical engineering applications.
Figures








Similar articles
-
Effect of amino acid sequence and pH on nanofiber formation of self-assembling peptides EAK16-II and EAK16-IV.Biomacromolecules. 2003 Sep-Oct;4(5):1433-42. doi: 10.1021/bm0341374. Biomacromolecules. 2003. PMID: 12959616
-
Effect of NaCl and peptide concentration on the self-assembly of an ionic-complementary peptide EAK16-II.Colloids Surf B Biointerfaces. 2005 Dec 20;46(3):152-61. doi: 10.1016/j.colsurfb.2005.11.004. Colloids Surf B Biointerfaces. 2005. PMID: 16321511
-
Effects of hydrophobicity and anions on self-assembly of the peptide EMK16-II.Biopolymers. 2010 Apr;93(4):318-29. doi: 10.1002/bip.21340. Biopolymers. 2010. PMID: 19885921
-
Self-Assembly of Homo Phenylalanine Oligopeptides: Role of Oligopeptide Chain Length.Langmuir. 2025 Jan 14;41(1):27-48. doi: 10.1021/acs.langmuir.4c03936. Epub 2025 Jan 2. Langmuir. 2025. PMID: 39745487 Review.
-
Peptide synthesis and self-assembly.Top Curr Chem. 2012;310:27-69. doi: 10.1007/128_2011_234. Top Curr Chem. 2012. PMID: 22025061 Review.
Cited by
-
Chemically-Induced Cross-Linking of Peptidic Fibrils for Scaffolding Polymeric Particles and Macrophages.Macromol Biosci. 2021 Apr;21(4):e2000350. doi: 10.1002/mabi.202000350. Epub 2021 Jan 27. Macromol Biosci. 2021. PMID: 33502824 Free PMC article.
-
Just how prevalent are peptide therapeutic products? A critical review.Int J Pharm. 2020 Sep 25;587:119491. doi: 10.1016/j.ijpharm.2020.119491. Epub 2020 Jul 3. Int J Pharm. 2020. PMID: 32622810 Free PMC article. Review.
-
Aβ8-20 Fragment as an Anti-Fibrillogenic and Neuroprotective Agent: Advancing toward Efficient Alzheimer's Disease Treatment.ACS Chem Neurosci. 2023 Mar 15;14(6):1126-1136. doi: 10.1021/acschemneuro.2c00720. Epub 2023 Mar 1. ACS Chem Neurosci. 2023. PMID: 36857606 Free PMC article.
-
RNA extraction from self-assembling peptide hydrogels to allow qPCR analysis of encapsulated cells.PLoS One. 2018 Jun 4;13(6):e0197517. doi: 10.1371/journal.pone.0197517. eCollection 2018. PLoS One. 2018. PMID: 29864116 Free PMC article.
-
All-atom molecular dynamics study of EAK16 peptide: the effect of pH on single-chain conformation, dimerization and self-assembly behavior.Eur Biophys J. 2014 May;43(4-5):143-55. doi: 10.1007/s00249-014-0949-x. Epub 2014 Mar 13. Eur Biophys J. 2014. PMID: 24623551
References
-
- Adamson, A. W., and A. P. Gast. 1997. Physical Chemistry of Surfaces, 6th ed. John Wiley & Sons, New York.
-
- Cabrerizo-Vilchez, M. A., Z. Policova, D. Y. Kwok, P. Chen, and A. W. Neumann. 1995. The temperature dependence of the interfacial tension of aqueous human albumin solution/decane. Colloids Surf. B Biointerfaces. 5:1–9.
-
- Campbell, N. A. 1993. Biology, 3rd ed. Benjamin/Cummings Publishing Company, Inc., Redwood City.
-
- Chen, P., S. Lahooti, Z. Policova, M. A. Cabrerizo-Vilchez, and A. W. Neumann. 1996. Concentration dependence of the film pressure of human serum albumin at the water/decane interface. Colloids Surf. B Biointerfaces. 6:279–289.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

