Tensile force-dependent neurite elicitation via anti-beta1 integrin antibody-coated magnetic beads
- PMID: 12829516
- PMCID: PMC1303117
- DOI: 10.1016/S0006-3495(03)74506-8
Tensile force-dependent neurite elicitation via anti-beta1 integrin antibody-coated magnetic beads
Erratum in
- Biophys J. 2004 Se[;87(3):623
Abstract
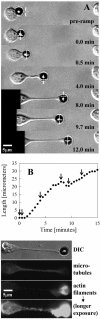
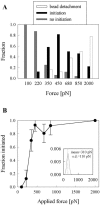
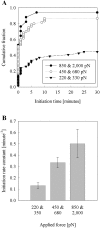
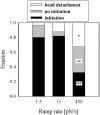
Previous work using glass microneedles to apply calibrated, localized force to neurons showed that tensile force is a sufficient signal for neurite initiation and elongation. However, previous studies did not examine the kinetics or probability of neurite initiation as a function of force or the rate of force application. Here we report the use of a new technique-magnetic bead force application-to systematically investigate the role of force in these phenomena with better ease of use and control over force than glass microneedles. Force-induced neurite initiation from embryonic chick forebrain neurons appeared to be a first-order random process whose rate increased with increasing force, and required the presence of peripheral microtubules. In addition, the probability of initiation was more than twofold lower for neurons exposed to rapid initial force ramps (450 pN/s) than for neurons exposed to slower ramps (1.5 and 11 pN/s). We observed a low force threshold for elongation (15-100 pN), which was not previously detected in chick forebrain neurites elongated by glass microneedles. Finally, neurites subjected to constant force elongated at variable instantaneous rates, and switched abruptly between elongation and retraction, similar to spontaneous, growth-cone-mediated outgrowth and microtubule dynamic instability.
Figures





Similar articles
-
Robust micromechanical neurite elicitation in synapse-competent neurons via magnetic bead force application.Ann Biomed Eng. 2005 Sep;33(9):1229-37. doi: 10.1007/s10439-005-5509-1. Ann Biomed Eng. 2005. PMID: 16133929
-
Mechanical tension as a regulator of axonal development.Neurotoxicology. 1994 Spring;15(1):95-107. Neurotoxicology. 1994. PMID: 8090366 Review.
-
Cytomechanics of neurite outgrowth from chick brain neurons.J Cell Sci. 1997 May;110 ( Pt 10):1179-86. doi: 10.1242/jcs.110.10.1179. J Cell Sci. 1997. PMID: 9191042
-
Magnetic microposts for mechanical stimulation of biological cells: fabrication, characterization, and analysis.Rev Sci Instrum. 2008 Apr;79(4):044302. doi: 10.1063/1.2906228. Rev Sci Instrum. 2008. PMID: 18447536 Free PMC article.
-
Cytomechanics of axonal development.Cell Biochem Biophys. 1995;27(3):135-55. doi: 10.1007/BF02738107. Cell Biochem Biophys. 1995. PMID: 9279454 Review.
Cited by
-
Parallelized Manipulation of Adherent Living Cells by Magnetic Nanoparticles-Mediated Forces.Int J Mol Sci. 2020 Sep 8;21(18):6560. doi: 10.3390/ijms21186560. Int J Mol Sci. 2020. PMID: 32911745 Free PMC article.
-
Magnetic tweezers-based force clamp reveals mechanically distinct apCAM domain interactions.Biophys J. 2012 Sep 19;103(6):1120-9. doi: 10.1016/j.bpj.2012.08.025. Biophys J. 2012. PMID: 22995484 Free PMC article.
-
Mechanical Regulation of Neurite Polarization and Growth: A Computational Study.Biophys J. 2020 Apr 21;118(8):1914-1920. doi: 10.1016/j.bpj.2020.02.031. Epub 2020 Mar 14. Biophys J. 2020. PMID: 32229314 Free PMC article.
-
Physical Forces in Regeneration of Cells and Tissues.Cold Spring Harb Perspect Biol. 2025 Apr 1;17(4):a041527. doi: 10.1101/cshperspect.a041527. Cold Spring Harb Perspect Biol. 2025. PMID: 38806241 Free PMC article. Review.
-
An integrated fiber-optic microfluidic device for detection of muscular force generation of microscopic nematodes.Lab Chip. 2012 Sep 21;12(18):3458-66. doi: 10.1039/c2lc40459a. Epub 2012 Jul 24. Lab Chip. 2012. PMID: 22824814 Free PMC article.
References
-
- Ahmad, F. J., J. Hughey, T. Wittmann, A. Hyman, M. Greaser, and P. W. Baas. 2000. Motor proteins regulate force interactions between microtubules and microfilaments in the axon. Nat. Cell Biol. 2:276–280. - PubMed
-
- Alenghat, F. J., B. Fabry, K. Y. Tsai, W. H. Goldmann, D. E. Ingber. 2000. Analysis of cell mechanics in single vinculin-deficient cells using a magnetic tweezer. Biochem. Res. Commun. 277:93–99. - PubMed
-
- Baas, P. W. 2002. Microtubule transport in the axon. Int. Rev. Cytol. 212:41–62. - PubMed
-
- Baas, P. W., and F. J. Ahmad. 2001. Force generation by cytoskeletal motor proteins as a regulator of axonal elongation and retraction. Trends Cell Biol. 11:244–249. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

