Nonvolatile read-out molecular memory
- PMID: 12829801
- PMCID: PMC166190
- DOI: 10.1073/pnas.0832270100
Nonvolatile read-out molecular memory
Abstract
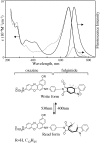
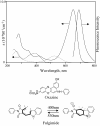
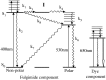
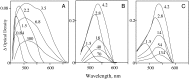
A versatile molecule is described that performs as a nondestructible read-out optical-storage molecular memory. This molecular memory is composed of two distinct molecules that are chemically bonded to each other to form a single molecule with unique properties. One component is a photochromic fulgimide, and the other is a strongly fluorescing oxazine dye. This composite molecule was specifically designed and synthesized to display, under specific conditions, both the photochromic functions of the first component and the fluorescence properties of the dye. To store information, the polar, closed form of the photochromic component is illuminated with 530-nm light, which converts it to the open, nonpolar form. The information is accessed by excitation at the 650-nm band of the oxazine dye component, causing the dye to fluoresce. However, the dye emits intense fluorescence under a nonpolar environment, which is attained only when the fulgimide component is in its open, nonpolar structure. The ultrafast kinetics, spectroscopy, and mechanism of the photoreaction of this molecule and its photoinduced intermediates have been measured, and fluorescence quantum yields and cross sections were determined.
Figures

References
-
- Parthenopoulos, D. & Rentzepis, P. M. (1989) Science 245 843%-845. - PubMed
-
- Dvornikov, A. S., Cokgor, I., Wang, M., McCormick, F. B., Esener, S. E. & Rentzepis, P. M. (1997) IEEE Trans. Compon. Packag. Manuf. Technol. Part A 20 200%-212.
-
- Dvornikov, A. S. & Rentzepis, P. M. (1997) Opt. Commun. 136 1%-6.
-
- Irie, M. (2000) Chem. Rev. (Washington, D.C.) 100 1685%-1716. - PubMed
-
- Kawata, S. & Kawata, Y. (2000) Chem. Rev. (Washington, D.C.) 100 1777%-1788. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

