Multiple interactions across the surface of the gp120 core structure determine the global neutralization resistance phenotype of human immunodeficiency virus type 1
- PMID: 12829845
- PMCID: PMC161940
- DOI: 10.1128/jvi.77.14.8061-8071.2003
Multiple interactions across the surface of the gp120 core structure determine the global neutralization resistance phenotype of human immunodeficiency virus type 1
Abstract
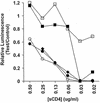
Resistance to neutralization is an important characteristic of primary isolates of human immunodeficiency virus type 1 (HIV-1) that relates to the potential for successful vaccination to prevent infection and use of immunotherapeutics for treatment of established infection. In order to further elucidate mechanisms responsible for neutralization resistance, we studied the molecular mechanisms that determine the resistance of the primary virus isolate of the strain HIV-1 MN to neutralization by soluble CD4 (sCD4). As is the case for the global neutralization resistance phenotype, sCD4 resistance depended upon sequences in the amino-terminal heptad repeat region of gp41 (HR1), as well as on multiple functional interactions within the envelope complex. The functional interactions that determined the resistance included interactions between the variable loop 1 and 2 (V1/V2) region and sequences in or near the CD4 binding site (CD4bs) and with the V3 loop. Additionally, the V3 loop region was found to interact functionally with sequences in the outer domain of gp120, distant from the CD4bs and coreceptor-binding site, as well as with a residue thought to be located centrally in the coreceptor-binding site. These and previous results provide the basis for a model by which functional signals that determine the neutralization resistance, high-infectivity phenotype depend upon interactions occurring across the surface of the gp120 core structure and involving variable loop structures and gp41. This model should be useful in efforts to define epitopes that may be important for primary virus neutralization.
Figures




References
-
- Bagley, J., P. J. Dillon, C. Rosen, J. Robinson, J. Sodroski, and W. A. Marasco. 1994. Structural characterization of broadly neutralizing human monoclonal antibodies against the CD4 binding site of HIV-1 gp120. Mol. Immunol. 31:1149-1160. - PubMed
-
- Barbas, C. F., J. E. Crowe, Jr., D. Cababa, T. M. Jones, S. L. Zebedee, B. R. Murphy, R. M. Chanock, and D. R. Burton. 1992. Human monoclonal Fab fragments derived from a combinatorial library bind to respiratory syncytial virus F glycoprotein and neutralize infectivity. Proc. Natl. Acad. Sci. USA 89:10164-10168. - PMC - PubMed
-
- Berman, P. W., T. J. Matthews, L. Riddle, M. Champe, M. R. Hobbs, G. R. Nakamura, J. Mercer, D. J. Eastman, C. Lucas, A. J. Langlois, F. M. Wurm, and T. J. Gregory. 1992. Neutralization of multiple laboratory and clinical isolates of human immunodeficiency virus type 1 (HIV-1) by antisera raised against gp120 from the MN isolate of HIV-1. J. Virol. 66:4464-4469. - PMC - PubMed
-
- Boots, L. J., P. M. McKenna, B. A. Arnold, P. M. Keller, M. K. Gorny, S. Zolla-Pazner, J. E. Robinson, and A. J. Conley. 1997. Anti-human immunodeficiency virus type 1 human monoclonal antibodies that bind discontinuous epitopes in the viral glycoproteins can identify mimotopes from recombinant phage peptide display libraries. AIDS Res. Hum. Retrovir. 13:1549-1559. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

