Human herpesvirus 7 open reading frame U12 encodes a functional beta-chemokine receptor
- PMID: 12829849
- PMCID: PMC161960
- DOI: 10.1128/jvi.77.14.8108-8115.2003
Human herpesvirus 7 open reading frame U12 encodes a functional beta-chemokine receptor
Abstract
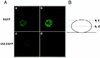
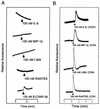
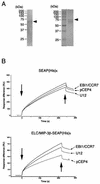
Human herpesvirus 7 (HHV-7), which belongs to the betaherpesvirus subfamily, infects mainly CD4+ T cells in vitro and infects children during infancy. After the primary infection, HHV-7 becomes latent. HHV-7 contains two genes (U12 and U51) that encode putative homologs of cellular G-protein-coupled receptors. To analyze the biological function of the U12 gene, we cloned the gene and expressed the U12 protein in cells. The U12 gene encoded a calcium-mobilizing receptor for the EBI1 ligand chemokine-macrophage inflammatory protein 3beta (ELC/MIP-3beta) but not for other chemokines, suggesting that the chemokine selectivity of the U12 gene product is distinct from that of the known mammalian chemokine receptors. These studies revealed that U12 activates distinct transmembrane signaling pathways that may mediate biological functions by binding with a beta-chemokine, ELC/MIP-3beta.
Figures




References
-
- Baba, M., T. Imai, M. Nishimura, M. Kakizaki, S. Takagi, K. Hieshima, H. Nomiyama, and O. Yoshie. 1997. Identification of CCR6, the specific receptor for a novel lymphocyte-directed CC chemokine LARC. J. Biol. Chem. 272:14893-14898. - PubMed
-
- Bais, C., B. Santomasso, O. Coso, L. Arvanitakis, E. G. Raaka, J. S. Gutkind, A. S. Asch, E. Cesarman, M. C. Gershengorn, E. A. Mesri, and M. C. Gerhengorn. 1998. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature 391:86-89. - PubMed
-
- Berneman, Z. N., D. V. Ablashi, G. Li, M. Eger-Fletcher, M. S. Reitz, Jr., C. L. Hung, I. Brus, A. L. Komaroff, and R. C. Gallo. 1992. Human herpesvirus 7 is a T-lymphotropic virus and is related to, but significantly different from, human herpesvirus 6 and human cytomegalovirus. Proc. Natl. Acad. Sci. USA 89:10552-10556. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

