Cooperative interactions of simian immunodeficiency virus Nef, AP-2, and CD3-zeta mediate the selective induction of T-cell receptor-CD3 endocytosis
- PMID: 12829850
- PMCID: PMC161955
- DOI: 10.1128/jvi.77.14.8116-8126.2003
Cooperative interactions of simian immunodeficiency virus Nef, AP-2, and CD3-zeta mediate the selective induction of T-cell receptor-CD3 endocytosis
Abstract
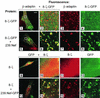
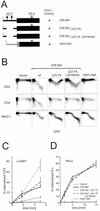
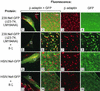
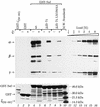
The Nef proteins of human immunodeficiency virus and simian immunodeficiency virus (SIV) bind the AP-1 and AP-2 clathrin adaptors to downmodulate the expression of CD4 and CD28 by recruiting them to sites of AP-2 clathrin-dependent endocytosis. Additionally, SIV Nef directly binds the CD3-zeta subunit of the CD3 complex and downmodulates the T-cell receptor (TCR)-CD3 complex. We report here that SIV mac239 Nef induces the endocytosis of TCR-CD3 in Jurkat T cells. SIV Nef also induces the endocytosis of a chimeric CD8-CD3-zeta protein containing only the CD3-zeta cytoplasmic domain (8-zeta), in the absence of other CD3 subunits. Thus, the interaction of SIV Nef with CD3-zeta likely mediates the induction of TCR-CD3 endocytosis. In cells expressing SIV Nef and 8-zeta, both proteins colocalize with AP-2, indicating that Nef induces 8-zeta internalization via this pathway. Surprisingly, deletion of constitutively strong AP-2 binding determinants (CAIDs) in SIV Nef had little effect on its ability to induce TCR-CD3, or 8-zeta endocytosis, even though these determinants are required for the induction of CD4 and CD28 endocytosis via this pathway. Fluorescent microscopic analyses revealed that while neither the mutant SIV Nef protein nor 8-zeta colocalized with AP-2 when expressed independently, both proteins colocalized with AP-2 when coexpressed. In vitro binding studies using recombinant SIV Nef proteins lacking CAIDs and recombinant CD3-zeta cytoplasmic domain demonstrated that SIV Nef and CD3-zeta cooperate to bind AP-2 via a novel interaction. The fact that Nef uses distinct AP-2 interaction surfaces to recruit specific membrane receptors demonstrates how Nef independently selects distinct types of target receptors and recruits them to AP-2 for endocytosis.
Figures

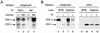
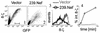
Similar articles
-
Two elements target SIV Nef to the AP-2 clathrin adaptor complex, but only one is required for the induction of CD4 endocytosis.EMBO J. 1999 May 17;18(10):2722-33. doi: 10.1093/emboj/18.10.2722. EMBO J. 1999. PMID: 10329619 Free PMC article.
-
The conserved process of TCR/CD3 complex down-modulation by SIV Nef is mediated by the central core, not endocytic motifs.Virology. 2002 Oct 10;302(1):106-22. doi: 10.1006/viro.2002.1628. Virology. 2002. PMID: 12429520
-
The T-cell receptor zeta chain contains two homologous domains with which simian immunodeficiency virus Nef interacts and mediates down-modulation.J Virol. 2000 Apr;74(7):3273-83. doi: 10.1128/jvi.74.7.3273-3283.2000. J Virol. 2000. PMID: 10708444 Free PMC article.
-
HIV and SIV Nef modulate signal transduction and protein sorting in T cells.Cold Spring Harb Symp Quant Biol. 1999;64:453-63. doi: 10.1101/sqb.1999.64.453. Cold Spring Harb Symp Quant Biol. 1999. PMID: 11232322 Review. No abstract available.
-
Role of Nef in primate lentiviral immunopathogenesis.Cell Mol Life Sci. 2008 Sep;65(17):2621-36. doi: 10.1007/s00018-008-8094-2. Cell Mol Life Sci. 2008. PMID: 18438604 Free PMC article. Review.
Cited by
-
Efficient Nef-mediated downmodulation of TCR-CD3 and CD28 is associated with high CD4+ T cell counts in viremic HIV-2 infection.J Virol. 2012 May;86(9):4906-20. doi: 10.1128/JVI.06856-11. Epub 2012 Feb 15. J Virol. 2012. PMID: 22345473 Free PMC article.
-
Tetherin downmodulation by SIVmac Nef lost with the H196Q escape variant is restored by an upstream variant.PLoS One. 2020 Aug 7;15(8):e0225420. doi: 10.1371/journal.pone.0225420. eCollection 2020. PLoS One. 2020. PMID: 32764749 Free PMC article.
-
Is the high virulence of HIV-1 an unfortunate coincidence of primate lentiviral evolution?Nat Rev Microbiol. 2009 Jun;7(6):467-76. doi: 10.1038/nrmicro2111. Epub 2009 Mar 23. Nat Rev Microbiol. 2009. PMID: 19305418 Review.
-
The SCHOOL of nature: IV. Learning from viruses.Self Nonself. 2010 Oct;1(4):282-298. doi: 10.4161/self.1.4.13279. Self Nonself. 2010. PMID: 21487503 Free PMC article.
-
A basic patch on alpha-adaptin is required for binding of human immunodeficiency virus type 1 Nef and cooperative assembly of a CD4-Nef-AP-2 complex.J Virol. 2009 Mar;83(6):2518-30. doi: 10.1128/JVI.02227-08. Epub 2009 Jan 7. J Virol. 2009. PMID: 19129443 Free PMC article.
References
-
- Aiken, C., J. Konner, N. R. Landau, M. E. Lenburg, and D. Trono. 1994. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76:853-864. - PubMed
-
- Bell, I., C. Ashman, J. Maughan, E. Hooker, F. Cook, and T. A. Reinhart. 1998. Association of simian immunodeficiency virus Nef with the T-cell receptor (TCR) zeta chain leads to TCR down-modulation. J. Gen. Virol. 79:2717-2727. - PubMed
-
- Bell, I., T. M. Schaefer, R. P. Trible, A. Amedee, and T. A. Reinhart. 2001. Down-modulation of the costimulatory molecule, CD28, is a conserved activity of multiple SIV Nefs and is dependent on histidine 196 of Nef. Virology 283:148-158. - PubMed
-
- Bresnahan, P. A., W. Yonemoto, S. Ferrell, D. Williams-Herman, R. Geleziunas, and W. C. Greene. 1998. A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr. Biol. 8:1235-1238. - PubMed
-
- Bresnahan, P. A., W. Yonemoto, and W. C. Greene. 1999. Cutting edge: SIV Nef protein utilizes both leucine- and tyrosine-based protein sorting pathways for down-regulation of CD4. J. Immunol. 163:2977-2981. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

