IRF-4,8 orchestrate the pre-B-to-B transition in lymphocyte development
- PMID: 12832394
- PMCID: PMC196178
- DOI: 10.1101/gad.1104803
IRF-4,8 orchestrate the pre-B-to-B transition in lymphocyte development
Abstract
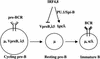
B-lymphocyte development involves sequential DNA rearrangements of immunoglobulin (Ig) heavy (mu) and light (kappa, lambda) chain loci and is dependent on transient expression of mu containing pre-antigen receptor complexes (pre-BCR). To date, genetic analysis has not identified transcription factors that coordinate the pre-B-to-B transition. We demonstrate that the related interferon regulatory factors IRF-4 (Pip) and IRF-8 (ICSBP) are required for Ig light but not heavy-chain gene rearrangement. In the absence of these transcription factors, B-cell development is arrested at the cycling pre-B-cell stage and the mutant cells fail to down-regulate the pre-BCR. On the basis of molecular analysis, we propose that IRF-4,8 function as a genetic switch to down-regulate surrogate light-chain gene expression and induce conventional light-chain gene transcription and rearrangement.
Figures



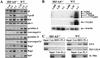
References
-
- Burrows P.D., Stephan, R.P., Wang, Y.H., Lassoued, K., Zhang, Z., and Cooper, M.D. 2002. The transient expression of pre-B cell receptors governs B cell development. Semin. Immunol. 14: 343–349. - PubMed
-
- Cheng A.M., Rowley, B., Pao, W., Hayday, A., Bolen, J.B., and Pawson, T. 1995. Syk tyrosine kinase required for mouse viability and B-cell development. Nature 378: 303–306. - PubMed
-
- DeKoter R.P., Lee, H.J., and Singh, H. 2002. PU.1 regulates expression of the interleukin-7 receptor in lymphoid progenitors. Immunity 16: 297–309. - PubMed
-
- Desiderio S., Lin, W.C., and Li, Z. 1996. The cell cycle and V(D)J recombination. Curr. Top. Microbiol. Immunol. 217: 45–59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
