Calcium signaling in single peripheral sensory nerve terminals
- PMID: 12832498
- PMCID: PMC6741185
- DOI: 10.1523/JNEUROSCI.23-12-04793.2003
Calcium signaling in single peripheral sensory nerve terminals
Abstract
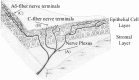
Peripheral sensory nerve terminals (PSNTs) have a dual function: reporting normal and abnormal sensations and releasing trophic factors to maintain the structure and function of epithelial cells. Although it is widely considered that intracellular Ca2+ plays a critical signaling role for both functions, the role of Ca2+ signaling has never been studied in PSNTs, primarily because of their small size and anatomical inaccessibility. Here, using epifluoresence microscopy and a fluorescent Ca2+ indicator, we report that action potentials or chemical irritation can elicit transient rises in [Ca2+]i (Ca2+ transients) in PSNTs within the corneal epithelium of the rat. In vitro electrical stimulation of the ciliary nerves in the eye, or electrical field stimulation of the cornea, evoked Ca2+ transients with a magnitude that was proportional to the number of stimuli applied over the range of 1-10 stimuli. Ca2+ transients were significantly blocked by 1 mm lidocaine, 4.1 microm saxitoxin (STX), or L-type Ca2+ channel antagonists (1 mm diltiazem or 20 microm nifedipine). The nociceptive agonist capsaicin (1 microm) elicited Ca2+ transients in all nerve terminals studied. Capsaicin-evoked Ca2+ transients were completely blocked by the vanilloid receptor 1 antagonist capsazepine (100 microm). In contrast, capsaicin-evoked Ca2+ transients were not attenuated by preincubation with 4.1 microm STX or 20 microm nifedipine. These findings demonstrate, for the first time, that nerve impulses or chemical stimulation promote Ca2+ entry into PSNTs, including nociceptors.
Figures



References
-
- Belmonte C, Gallar J ( 1996) Corneal nociceptors. In: Neurobiology of nociceptors (Belmonte C, Cervero F, eds), pp 146–183. New York: Oxford UP.
-
- Black JA, Waxman SG ( 2002) Molecular identities of two tetrodotoxin-resistant sodium channels in corneal axons. Exp Eye Res 75: 193–199. - PubMed
-
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D ( 1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 398: 816–824. - PubMed
-
- Chen X, Belmonte C, Rang HP ( 1997a) Capsaicin and carbon dioxide act by distinct mechanisms on sensory nerve terminals in the cat cornea. Pain 70: 23–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
