Early maintenance of hippocampal mossy fiber--long-term potentiation depends on protein and RNA synthesis and presynaptic granule cell integrity
- PMID: 12832506
- PMCID: PMC6741163
- DOI: 10.1523/JNEUROSCI.23-12-04842.2003
Early maintenance of hippocampal mossy fiber--long-term potentiation depends on protein and RNA synthesis and presynaptic granule cell integrity
Abstract
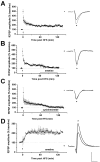
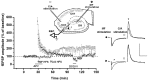
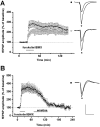
The neural substrates of memory likely include long-term potentiation (LTP) of synaptic strength that results from high-frequency stimulation (HFS) of the afferent pathway. The mechanisms that underlie the maintenance of LTP include RNA and protein synthesis, although the contribution of these molecular events typically does not become essential until several hours after LTP induction. We here show that, different from this pattern, (1) LTP maintenance at the mossy fiber (MF) input to CA3 pyramidal cells in the hippocampus depends on protein and RNA synthesis soon after LTP induction, and (2) some of these molecular events are controlled by signaling from the presynaptic granule cell soma. Bath application of the protein synthesis inhibitor emetine or cycloheximide 1 hr after MF LTP induction in hippocampal slices caused loss of MF potentiation. In contrast, application of emetine 1 hr after LTP induction at the commissural-associational input to CA3 pyramidal cells had no effect on this form of LTP. Administration of emetine or the RNA synthesis inhibitor actinomycin-D before delivery of HFS to MF input also caused a rapid decay of MF potentiation, although neither drug had an effect on the amplitude or the time-constant of decay of post-tetanic potentiation (PTP). Similarly, transection of MF axons near granule cell somas had no effect on baseline or PTP parameters but caused loss of potentiation at a rate comparable with that after actinomycin-D application. These results indicate that the mechanisms that underlie MF LTP maintenance differ from those involved in LTP maintenance at other glutamatergic synapses.
Figures


References
-
- Aakalu G, Smith WB, Nguyen N, Jiang C, Schuman EM ( 2001) Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron 30: 489–502. - PubMed
-
- Bennet MR ( 2000) The concept of long term potentiation of transmission at synapse. Prog Neurobiol 60: 109–137. - PubMed
-
- Brown A ( 2000) Slow axonal transport: stop and go traffic in the axon. Nat Rev Mol Cell Biol 1: 153–156. - PubMed
-
- Castillo EP, Janz R, Südhof TC, Tzounopoulos T, Malenka RC, Nicoll RA ( 1997) Rab3A is essential for mossy fibre long-term potentiation in the hippocampus. Nature 388: 590–593. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
