NR2B and NR2D subunits coassemble in cerebellar Golgi cells to form a distinct NMDA receptor subtype restricted to extrasynaptic sites
- PMID: 12832518
- PMCID: PMC6741215
- DOI: 10.1523/JNEUROSCI.23-12-04958.2003
NR2B and NR2D subunits coassemble in cerebellar Golgi cells to form a distinct NMDA receptor subtype restricted to extrasynaptic sites
Abstract
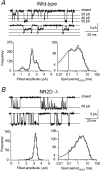
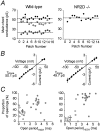
NMDA receptors (NMDARs) are thought to be tetrameric assemblies composed of NR1 and at least one type of NR2 subunit. The identity of the NR2 subunit (NR2A, -B, -C, -D) is critical in determining many of the functional properties of the receptor, such as channel conductance and deactivation time. Further diversity may arise from coassembly of more than one type of NR2 subunit, if the resulting triheteromeric assembly (NR1 plus two types of NR2) displays distinct functional properties. We have used gene-ablated mice (NR2D -/-) to examine the effects of the NR2D subunit on NMDAR channels and NMDAR EPSCs in cerebellar Golgi cells. These cells are thought to express both NR2B and NR2D subunits, a combination that occurs widely in the developing nervous system. Our experiments provide direct evidence that the low conductance NMDAR channels in Golgi cells arise from diheteromeric NR1/NR2D assemblies. To investigate whether a functionally distinct triheteromeric assembly was also expressed, we analyzed the kinetic and pharmacological properties of single-channel currents in isolated extrasynaptic patches. We found that after the loss of the NR2D subunit, the properties of the 50 pS NMDAR channels were altered. This result is consistent with the presence of a triheteromeric assembly (NR1/NR2B/NR2D) in cells from wild-type mice. However, we could find no difference in the properties of NMDAR-mediated EPSCs between wild-type and NR2D subunit ablated mice. Our experiments suggest that although both diheteromeric and triheteromeric NR2D-containing receptors are expressed in cerebellar Golgi cells, neither receptor type participates in parallel fiber to Golgi cell synaptic transmission. The presence of the NR2D subunit within an assembly may therefore result in its restriction to extrasynaptic sites.
Figures



References
-
- Akazawa C, Shigemoto R, Bessho Y, Nakanishi S, Mizuno N ( 1994) Differential expression of five N-methyl-d-aspartate receptor subunit mRNAs in the cerebellum of developing and adult rats. J Comp Neurol 347: 150–160. - PubMed
-
- Barria A, Malinow R ( 2002) Subunit-specific NMDA receptor trafficking to synapses. Neuron 35: 345–353. - PubMed
-
- Béhé P, Stern P, Wyllie DJ, Nassar M, Schoepfer R, Colquhoun D ( 1995) Determination of NMDA NR1 subunit copy number in recombinant NMDA receptors. Proc R Soc Lond B Biol Sci 262: 205–213. - PubMed
-
- Chazot PL, Stephenson FA ( 1997) Molecular dissection of native mammalian forebrain NMDA receptors containing the NR1 C2 exon: direct demonstration of NMDA receptors comprising NR1, NR2A, and NR2B subunits within the same complex. J Neurochem 69: 2138–2144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
