Human embryonic germ cell derivatives facilitate motor recovery of rats with diffuse motor neuron injury
- PMID: 12832537
- PMCID: PMC6741166
- DOI: 10.1523/JNEUROSCI.23-12-05131.2003
Human embryonic germ cell derivatives facilitate motor recovery of rats with diffuse motor neuron injury
Abstract
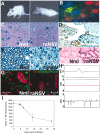
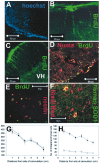
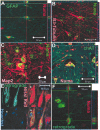
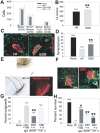
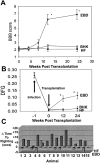
We have investigated the potential of human pluripotent cells to restore function in rats paralyzed with a virus-induced motor neuronopathy. Cells derived from embryonic germ cells, termed embryoid body-derived (EBD) cells, introduced into the CSF were distributed extensively over the rostrocaudal length of the spinal cord and migrated into the spinal cord parenchyma in paralyzed, but not uninjured, animals. Some of the transplanted human cells expressed the neuroglial progenitor marker nestin, whereas others expressed immunohistochemical markers characteristic of astrocytes or mature neurons. Rare transplanted cells developed immunoreactivity to choline acetyltransferase (ChAT) and sent axons into the sciatic nerve as detected by retrograde labeling. Paralyzed animals transplanted with EBD cells partially recovered motor function 12 and 24 weeks after transplantation, whereas control animals remained paralyzed. Semi-quantitative analysis revealed that the efficiency of neuronal differentiation and extension of neurites could not account for the functional recovery. Rather, transplanted EBD cells protected host neurons from death and facilitated reafferentation of motor neuron cell bodies. In vitro, EBD cells secrete transforming growth factor-alpha (TGF-alpha) and brain-derived neurotrophic factor (BDNF). Neutralizing antibodies to TGF-alpha and to BDNF abrogated the ability of EBD-conditioned media to sustain motor neuron survival in culture, whereas neutralizing antibodies to BDNF eliminated the axonal outgrowth from spinal organotypics observed with direct coculture of EBD cells. We conclude that cells derived from human pluripotent stem cells have the capacity to restore neurologic function in animals with diffuse motor neuron disease via enhancement of host neuron survival and function.
Figures
Similar articles
-
Functional recovery after human umbilical cord blood cells transplantation with brain-derived neutrophic factor into the spinal cord injured rat.Acta Neurochir (Wien). 2005 Sep;147(9):985-92; discussion 992. doi: 10.1007/s00701-005-0538-y. Epub 2005 Jul 11. Acta Neurochir (Wien). 2005. PMID: 16010451
-
Neural progenitor cells enhance the survival and axonal regeneration of injured motoneurons after transplantation into the avulsed ventral horn of adult rats.J Neurotrauma. 2009 Jan;26(1):67-80. doi: 10.1089/neu.2008.0656. J Neurotrauma. 2009. PMID: 19196181
-
In vitro neuronal differentiation of cultured human embryonic germ cells.Biochem Biophys Res Commun. 2005 Feb 11;327(2):548-56. doi: 10.1016/j.bbrc.2004.11.168. Biochem Biophys Res Commun. 2005. PMID: 15629148
-
Generation of motor neurons from pluripotent stem cells.Prog Brain Res. 2012;201:313-31. doi: 10.1016/B978-0-444-59544-7.00015-9. Prog Brain Res. 2012. PMID: 23186721 Review.
-
Neuronal apoptosis pathways in Sindbis virus encephalitis.Prog Mol Subcell Biol. 2004;36:71-93. doi: 10.1007/978-3-540-74264-7_5. Prog Mol Subcell Biol. 2004. PMID: 15171608 Review.
Cited by
-
Convection enhanced delivery for treating brain tumors and selected neurological disorders: symposium review.J Neurooncol. 2007 May;83(1):97-109. doi: 10.1007/s11060-006-9308-9. Epub 2007 Jan 4. J Neurooncol. 2007. PMID: 17203397 Review.
-
Microdialysis in central nervous system disorders and their treatment.Pharmacol Biochem Behav. 2008 Aug;90(2):282-96. doi: 10.1016/j.pbb.2008.03.001. Epub 2008 Mar 10. Pharmacol Biochem Behav. 2008. PMID: 18436292 Free PMC article. Review.
-
In vitro immunogenicity of undifferentiated pluripotent stem cells (PSC) and derived lineages.Semin Immunopathol. 2011 Nov;33(6):551-62. doi: 10.1007/s00281-011-0265-9. Epub 2011 Apr 5. Semin Immunopathol. 2011. PMID: 21461990 Review.
-
Spinal muscular atrophy: advances in research and consensus on care of patients.Curr Treat Options Neurol. 2008 Nov;10(6):420-8. doi: 10.1007/s11940-008-0044-7. Curr Treat Options Neurol. 2008. PMID: 18990310
-
Present state and future perspectives of using pluripotent stem cells in toxicology research.Arch Toxicol. 2011 Feb;85(2):79-117. doi: 10.1007/s00204-010-0641-6. Epub 2011 Jan 12. Arch Toxicol. 2011. PMID: 21225242 Free PMC article. Review.
References
-
- Barde YA, Davies AM, Johnson JE, Lindsay RM, Thoenen H ( 1987) Brain-derived neurotrophic factor. Prog Brain Res 71: 185–189. - PubMed
-
- Basso DM, Beattie MS, Bresnahan JC ( 1995) A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12: 1–21. - PubMed
-
- Benedetti S, Pirola B, Pollo B, Magrassi L, Bruzzone MG, Rigamonti D, Galli R, Selleri S, Di Meco F, De Fraja C, Vescovi A, Cattaneo E, Finocchiaro G ( 2000) Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med 6: 447–450. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
