Endogenously produced neurotrophins regulate survival and differentiation of cortical progenitors via distinct signaling pathways
- PMID: 12832539
- PMCID: PMC6741181
- DOI: 10.1523/JNEUROSCI.23-12-05149.2003
Endogenously produced neurotrophins regulate survival and differentiation of cortical progenitors via distinct signaling pathways
Abstract
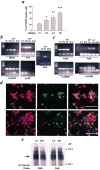
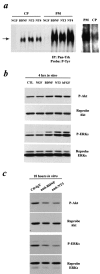
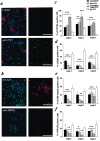
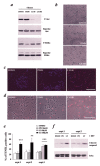
Cultured embryonic cortical progenitor cells will mimic the temporal differentiation pattern observed in vivo, producing neurons first and then glia. Here, we investigated the role of two endogenously produced growth factors, the neurotrophins brain-derived neurotrophic factor and neurotrophin-3 (NT-3), in the early progenitor-to-neuron transition. Cultured cortical progenitors express BDNF and NT-3, as well as their receptors TrkB (tyrosine kinase receptor B) and TrkC. Inhibition of these endogenously expressed neurotrophins using function-blocking antibodies resulted in a marked decrease in the survival of cortical progenitors, accompanied by decreased proliferation and inhibition of neurogenesis. Inhibition of neurotrophin function also suppressed the downstream Trk receptor signaling pathways, PI3-kinase (phosphatidyl inositol-3-kinase) and MEK-ERK (MAP kinase kinase-extracellular signal-regulated kinase), indicating the presence of autocrine-paracrine neurotrophin:Trk receptor signaling in these cells. Moreover, specific inhibition of these two Trk signaling pathways led to distinct biological effects; inhibition of PI3-kinase decreased progenitor cell survival, whereas inhibition of MEK selectively blocked the generation of neurons, with no effects on survival or proliferation. Thus, neurotrophins made by cortical progenitor cells themselves signal through the TrkB and TrkC receptors to mediate cortical progenitor cell survival and neurogenesis via two distinct downstream signaling pathways.
Figures


References
-
- Antonopoulos J, Pappas IS, Parnavelas JG ( 1997) Activation of the GABAA receptor inhibits the proliferative effects of bFGF in cortical progenitor cells. Eur J Neurosci 9: 291–298. - PubMed
-
- Benoit BO, Savarese T, Joly M, Engstrom CM, Pang L, Reilly J, Recht LD, Ross AH, Quesenberry PJ ( 2001) Neurotrophin channeling of neural progenitor cell differentiation. J Neurobiol 46: 265–280. - PubMed
-
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME ( 1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96: 857–868. - PubMed
-
- Burrows RC, Wancio D, Levitt P, Lillien L ( 1997) Response diversity and the timing of progenitor cell maturation are regulated by developmental changes in EGFR expression in the cortex. Neuron 19: 251–267. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
