Increased depression-like behaviors in corticotropin-releasing factor receptor-2-deficient mice: sexually dichotomous responses
- PMID: 12832554
- PMCID: PMC6741155
- DOI: 10.1523/JNEUROSCI.23-12-05295.2003
Increased depression-like behaviors in corticotropin-releasing factor receptor-2-deficient mice: sexually dichotomous responses
Abstract
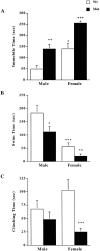
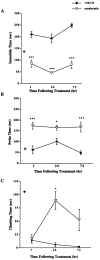
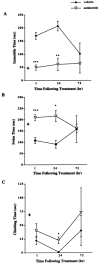
Depressive disorders affect nearly 19 million American adults, making depression and the susceptibility for developing depression a critical focus of mental health research today. Females are twice as likely to develop depression as males. Stress is a known risk factor for developing depression, and recent hypotheses suggest an involvement of an overactive stress axis. As mediators of the stress response, corticotropin-releasing factor (CRF) and its receptors (CRFR1 and CRFR2) have been implicated in the propensity for developing stress-related mood disorders. Mice deficient in CRFR2 display increased anxiety-like behaviors and a hypersensitive stress response. As a possible animal model of depression, these mice were tested for depression-like behaviors in the forced swim test. Comparisons were made between wild-type and mutant animals, as well as between sexes. Male and female CRFR2-mutant mice showed increased immobility as an indicator of depression compared with wild-type mice of the same sex. In addition, mutant and wild-type female mice demonstrated increased immobile time compared with males of the same genotype. Treatment of CRFR2-deficient mice with the CRFR1 antagonist antalarmin decreased immobile time and increased swim time in both sexes. We found a significant effect of sex for both time spent immobile and swimming after antalarmin treatment. Because differences in behaviors in the forced swim test are good indicators of serotonergic and catecholaminergic involvement, our results may reveal an interaction of CRF pathways with other known antidepressant systems and may also support an involvement of CRF receptors in the development of depression such that elevated CRFR1 activity, in the absence of CRFR2, increases depression-like behaviors.
Figures
References
-
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB ( 1999) The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol 160: 1–12. - PubMed
-
- Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF ( 2000) Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet 24: 410–414. - PubMed
-
- Coste SC, Kesterson RA, Heldwein KA, Stevens SL, Heard AD, Hollis JH, Murray SE, Hill JK, Pantely GA, Hohimer AR, Hatton DC, Phillips TJ, Finn DA, Low MJ, Rittenberg MB, Stenzel P, Stenzel-Poore MP ( 2000) Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet 24: 403–409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
