Engineered chromosome regions with altered sequence composition demonstrate hierarchical large-scale folding within metaphase chromosomes
- PMID: 12835314
- PMCID: PMC2172725
- DOI: 10.1083/jcb.200303098
Engineered chromosome regions with altered sequence composition demonstrate hierarchical large-scale folding within metaphase chromosomes
Abstract
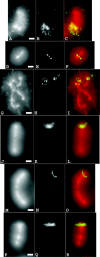
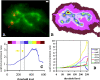
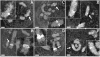
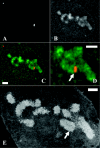
Mitotic chromosome structure and DNA sequence requirements for normal chromosomal condensation remain unknown. We engineered labeled chromosome regions with altered scaffold-associated region (SAR) sequence composition as a formal test of the radial loop and other chromosome models. Chinese hamster ovary cells were isolated containing high density insertions of a transgene containing lac operator repeats and a dihydrofolate reductase gene, with or without flanking SAR sequences. Lac repressor staining provided high resolution labeling with good preservation of chromosome ultrastructure. No evidence emerged for differential targeting of SAR sequences to a chromosome axis within native chromosomes. SAR sequences distributed uniformly throughout the native chromosome cross section and chromosome regions containing a high density of SAR transgene insertions showed normal diameter and folding. Ultrastructural analysis of two different transgene insertion sites, both spanning less than the full chromatin width, clearly contradicted predictions of simple radial loop models while providing strong support for hierarchical models of chromosome architecture. Specifically, an approximately 250-nm-diam folding subunit was visualized directly within fully condensed metaphase chromosomes. Our results contradict predictions of simple radial loop models and provide the first unambiguous demonstration of a hierarchical folding subunit above the level of the 30-nm fiber within normally condensed metaphase chromosomes.
Figures



Similar articles
-
Visualization of early chromosome condensation: a hierarchical folding, axial glue model of chromosome structure.J Cell Biol. 2004 Sep 13;166(6):775-85. doi: 10.1083/jcb.200406049. Epub 2004 Sep 7. J Cell Biol. 2004. PMID: 15353545 Free PMC article.
-
Mitotic chromosome structure: reproducibility of folding and symmetry between sister chromatids.Biophys J. 2009 Feb 18;96(4):1617-28. doi: 10.1016/j.bpj.2008.10.051. Biophys J. 2009. PMID: 19217877 Free PMC article.
-
Electron microscopy and atomic force microscopy studies of chromatin and metaphase chromosome structure.Micron. 2011 Dec;42(8):733-50. doi: 10.1016/j.micron.2011.05.002. Epub 2011 May 12. Micron. 2011. PMID: 21703860 Review.
-
In vivo localization of DNA sequences and visualization of large-scale chromatin organization using lac operator/repressor recognition.J Cell Biol. 1996 Dec;135(6 Pt 2):1685-700. doi: 10.1083/jcb.135.6.1685. J Cell Biol. 1996. PMID: 8991083 Free PMC article.
-
Mitotic chromosome structure.Exp Cell Res. 2012 Jul 15;318(12):1381-5. doi: 10.1016/j.yexcr.2012.03.027. Epub 2012 Apr 4. Exp Cell Res. 2012. PMID: 22507271 Review.
Cited by
-
Evidence of activity-specific, radial organization of mitotic chromosomes in Drosophila.PLoS Biol. 2011 Jan 11;9(1):e1000574. doi: 10.1371/journal.pbio.1000574. PLoS Biol. 2011. PMID: 21264350 Free PMC article.
-
Stacked thin layers of metaphase chromatin explain the geometry of chromosome rearrangements and banding.Sci Rep. 2015 Oct 8;5:14891. doi: 10.1038/srep14891. Sci Rep. 2015. PMID: 26446309 Free PMC article.
-
The chromosome folding problem and how cells solve it.Cell. 2024 Nov 14;187(23):6424-6450. doi: 10.1016/j.cell.2024.10.026. Cell. 2024. PMID: 39547207 Free PMC article. Review.
-
The 3D Topography of Mitotic Chromosomes.Mol Cell. 2020 Sep 17;79(6):902-916.e6. doi: 10.1016/j.molcel.2020.07.002. Epub 2020 Aug 7. Mol Cell. 2020. PMID: 32768407 Free PMC article.
-
Structure of metaphase chromosomes: a role for effects of macromolecular crowding.PLoS One. 2012;7(4):e36045. doi: 10.1371/journal.pone.0036045. Epub 2012 Apr 23. PLoS One. 2012. PMID: 22540018 Free PMC article.
References
-
- Barsoum, J. 1990. Introduction of stable high-copy-number DNA into Chinese hamster ovary cells by electroporation. DNA Cell Biol. 9:293–300. - PubMed
-
- Baumgartner, M., B. Dutrillaux, N. Lemieux, A. Lilienbaum, D. Paulin, and E. Viegas-Pequignot. 1991. Genes occupy a fixed and symmetrical position on sister chromatids. Cell. 64:761–766. - PubMed
-
- Belmont, A.S. 1997. Large-scale chromatin organization. Genome Structure and Function. C. Nicolini, editor. Kluwer Academic Publishers, Dordrecht, Netherlands. 261–276.
-
- Belmont, A.S., M.B. Braunfeld, J.W. Sedat, and D.A. Agard. 1989. Large-scale chromatin structural domains within mitotic and interphase chromosomes in vivo and in vitro. Chromosoma. 98:129–143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

