Calcium clearance mechanisms of mouse sperm
- PMID: 12835474
- PMCID: PMC2234473
- DOI: 10.1085/jgp.200308839
Calcium clearance mechanisms of mouse sperm
Erratum in
- J Gen Physiol. 2003 Sep;122(3):375
Abstract
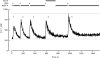
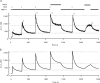
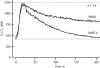
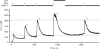
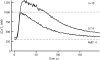
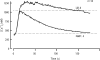
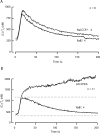
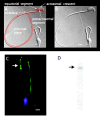
The spermatozoon is specialized for a single vital role in fertilization. Past studies show that Ca2+ signals produced by the opening of plasma membrane entry channels initiate several events required for the sperm to reach and enter the egg but reveal little about how resting [Ca2+]i is maintained or restored after elevation. We examined these homeostatic mechanisms by monitoring the kinetics of recovery from depolarizing stimuli under conditions intended to inhibit candidate mechanisms for sequestration or extrusion of Ca2+ from the cytosol. We found that the Ca2+-ATPase pump of the plasma membrane performs the major task of Ca2+ clearance. It is essential in the final stages of recovery to achieve a low resting [Ca2+]i. With immunomethods we found a approximately 130-kD plasma membrane Ca2+-ATPase protein on Western blots of whole sperm extracts and showed immunolocalization to the proximal principal piece of the flagellum. The plasma membrane Na+-Ca2+ exchanger also exports Ca2+ when [Ca2+]i is elevated. Simultaneous inhibition of both mechanisms of extrusion revealed an additional contribution to clearance from a CCCP-sensitive component, presumably sequestration by the mitochondria. Involvement of SERCA pumps was not clearly detected. Many aspects of the kinetics of Ca2+ clearance observed in the presence and absence of inhibitors were reproduced in a mathematical model based on known and assumed kinetic parameters. The model predicts that when cytosolic [Ca2+] is at 1 microM, the rates of removal by the Ca2+-ATPase, Na+-Ca2+-exchanger, mitochondrial uniporter, and SERCA pump are approximately 1.0, 0.35, 0.33, and 0 micromole l(-1) s(-1), rates substantially slower than those reported for other cells studied by similar methods. According to the model, the Na+-Ca2+ exchanger is poised so that it may run in reverse at resting [Ca2+]i levels. We conclude that the essential functions of sperm do not require the ability to recover rapidly from globally elevated cytosolic [Ca2+].
Figures
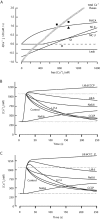
References
-
- Adeoya-Osiguwa, S.A., and L.R. Fraser. 1996. Evidence for Ca2+-dependent ATPase activity, stimulated by decapacitation factor and calmodulin, in mouse sperm. Mol. Reprod. Dev. 44:111–120. - PubMed
-
- Babcock, D.F., N.L. First, and H.A. Lardy. 1976. Action of ionophore A23187 at the cellular level: Separation of effects at the plasma and mitochondrial membranes. J. Biol. Chem. 251:3881–3886. - PubMed
-
- Babcock, D.F. 1983. Examination of the intracellular ionic environment and of ionophore action by null point measurements employing the fluorescein chromophore. J. Biol. Chem. 258:6380–6389. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

