Presentation of exogenous antigens on major histocompatibility complex (MHC) class I and MHC class II molecules is differentially regulated during dendritic cell maturation
- PMID: 12835477
- PMCID: PMC2196081
- DOI: 10.1084/jem.20021542
Presentation of exogenous antigens on major histocompatibility complex (MHC) class I and MHC class II molecules is differentially regulated during dendritic cell maturation
Abstract
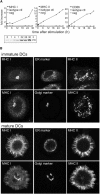
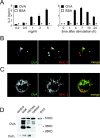
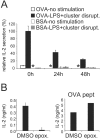
During maturation, dendritic cells (DCs) regulate their capacity to process and present major histocompatibility complex (MHC) II-restricted antigens. Here we show that presentation of exogenous antigens by MHC I is also subject to developmental control, but in a fashion strikingly distinct from MHC II. Immature mouse bone marrow-derived DCs internalize soluble ovalbumin and sequester the antigen intracellularly until they receive an appropriate signal that induces cross presentation. At that time, peptides are generated in a proteasome-dependent fashion and used to form peptide-MHC I complexes that appear at the plasma membrane. Unlike MHC II, these events do not involve a marked redistribution of preexisting MHC I molecules from intracellular compartments to the DC surface. Moreover, out of nine stimuli well known to induce the phenotypic maturation of DCs and to promote MHC II presentation, only two (CD40 ligation, disruption of cell-cell contacts) activated cross presentation on MHC I. In contrast, formation of peptide-MHC I complexes from endogenous cytosolic antigens occurs even in unstimulated, immature DCs. Thus, the MHC I and MHC II pathways of antigen presentation are differentially regulated during DC maturation.
Figures





Similar articles
-
The formation of immunogenic major histocompatibility complex class II-peptide ligands in lysosomal compartments of dendritic cells is regulated by inflammatory stimuli.J Exp Med. 2000 Mar 20;191(6):927-36. doi: 10.1084/jem.191.6.927. J Exp Med. 2000. PMID: 10727455 Free PMC article.
-
Auranofin, an immunosuppressive drug, inhibits MHC class I and MHC class II pathways of antigen presentation in dendritic cells.Arch Pharm Res. 2008 Mar;31(3):370-6. doi: 10.1007/s12272-001-1166-9. Epub 2008 Apr 13. Arch Pharm Res. 2008. PMID: 18409052
-
Regulation of MHC class I transport in human dendritic cells and the dendritic-like cell line KG-1.J Immunol. 2003 Apr 15;170(8):4178-88. doi: 10.4049/jimmunol.170.8.4178. J Immunol. 2003. PMID: 12682250
-
Control of MHC class II antigen presentation in dendritic cells: a balance between creative and destructive forces.Immunol Rev. 2005 Oct;207:191-205. doi: 10.1111/j.0105-2896.2005.00317.x. Immunol Rev. 2005. PMID: 16181337 Review.
-
Capture and processing of exogenous antigens for presentation on MHC molecules.Annu Rev Immunol. 1997;15:821-50. doi: 10.1146/annurev.immunol.15.1.821. Annu Rev Immunol. 1997. PMID: 9143708 Review.
Cited by
-
Whole lymphoma B cells allow efficient cross-presentation of antigens by dendritic cells.Cytotherapy. 2008;10(6):642-9. doi: 10.1080/14653240802317647. Cytotherapy. 2008. PMID: 18836919 Free PMC article.
-
Intracellular regulation of cross-presentation during dendritic cell maturation.PLoS One. 2013 Oct 3;8(10):e76801. doi: 10.1371/journal.pone.0076801. eCollection 2013. PLoS One. 2013. PMID: 24098562 Free PMC article.
-
Inflammatory spleen monocytes can upregulate CD11c expression without converting into dendritic cells.J Immunol. 2012 Apr 15;188(8):3603-10. doi: 10.4049/jimmunol.1102741. Epub 2012 Mar 21. J Immunol. 2012. PMID: 22442444 Free PMC article.
-
Intracellular Transport Routes for MHC I and Their Relevance for Antigen Cross-Presentation.Front Immunol. 2015 Jul 2;6:335. doi: 10.3389/fimmu.2015.00335. eCollection 2015. Front Immunol. 2015. PMID: 26191062 Free PMC article. Review.
-
Cell Signaling Pathways That Regulate Antigen Presentation.J Immunol. 2016 Oct 15;197(8):2971-2979. doi: 10.4049/jimmunol.1600460. J Immunol. 2016. PMID: 27824592 Free PMC article. Review.
References
-
- Rock, K.L., I.A. York, T. Saric, and A.L. Goldberg. 2002. Protein degradation and the generation of MHC class I-presented peptides. Adv. Immunol. 80:1–70. - PubMed
-
- Yewdell, J.W., C.C. Norbury, and J.R. Bennink. 1999. Mechanisms of exogenous antigen presentation by MHC class I molecules in vitro and in vivo: implications for generating CD8+ T cell responses to infectious agents, tumors, transplants, and vaccines. Adv. Immunol. 73:1–77. - PubMed
-
- Sigal, L.J., S. Crotty, R. Andino, and K.L. Rock. 1999. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature. 398:77–80. - PubMed
-
- Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. - PubMed
-
- Norbury, C.C., B.J. Chambers, A.R. Prescott, H.G. Ljunggren, and C. Watts. 1997. Constitutive macropinocytosis allows TAP-dependent major histocompatibility complex class I presentation of exogenous soluble antigen by bone marrow-derived dendritic cells. Eur. J. Immunol. 27:280–288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

