A microscopic view of ion conduction through the K+ channel
- PMID: 12837936
- PMCID: PMC166365
- DOI: 10.1073/pnas.1431750100
A microscopic view of ion conduction through the K+ channel
Abstract
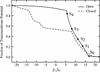
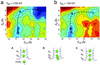
Recent results from x-ray crystallography and molecular dynamics free-energy simulations have revealed the existence of a number of specific cation-binding sites disposed along the narrow pore of the K+ channel from Streptomyces lividans (KcsA), suggesting that K+ ions might literally "hop" in single file from one binding site to the next as permeation proceeds. In support of this view, it was found that the ion configurations correspond to energy wells of similar depth and that ion translocation is opposed only by small energy barriers. Although such features of the multiion potential energy surface are certainly essential for achieving a high throughput rate, diffusional and dissipative dynamical factors must also be taken into consideration to understand how rapid conduction of K+ is possible. To elucidate the mechanism of ion conduction, we established a framework theory enabling the direct simulation of nonequilibrium fluxes by extending the results of molecular dynamics over macroscopically long times. In good accord with experimental measurements, the simulated maximum conductance of the channel at saturating concentration is on the order of 550 and 360 pS for outward and inward ions flux, respectively, with a unidirectional flux-ratio exponent of 3. Analysis of the ion-conduction process reveals a lack of equivalence between the cation-binding sites in the selectivity filter.
Figures

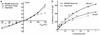
References
-
- Hille, B. (2001) Ionic Channels of Excitable Membranes (Sinauer, Sunderland, MA), 3rd Ed.
-
- Zhou, Y., Morais-Cabral, J. H., Kaufman, A. & MacKinnon, R. (2001) Nature 414, 43–48. - PubMed
-
- Doyle, D. A., Cabral, J. M., Pfuetzner, R. A., Kuo, A., Gulbis, J. M., Cohen, S. L., Chait, B. T. & MacKinnon, R. (1998) Science 280, 69–77. - PubMed
-
- Jiang, Y., Lee, A., Chen, J., Cadene, M., Chait, B. T. & MacKinnon, R. (2002) Nature 417, 515–522. - PubMed
-
- Bernèche, S. & Roux, B. (2001) Nature 414, 73–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

