Sexual and apomictic reproduction in Hieracium subgenus pilosella are closely interrelated developmental pathways
- PMID: 12837944
- PMCID: PMC165398
- DOI: 10.1105/tpc.011742
Sexual and apomictic reproduction in Hieracium subgenus pilosella are closely interrelated developmental pathways
Abstract
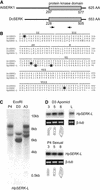
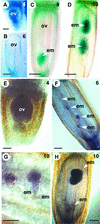
Seed formation in flowering plants requires meiosis of the megaspore mother cell (MMC) inside the ovule, selection of a megaspore that undergoes mitosis to form an embryo sac, and double fertilization to initiate embryo and endosperm formation. During apomixis, or asexual seed formation, in Hieracium ovules, a somatic aposporous initial (AI) cell divides to form a structurally variable aposporous embryo sac and embryo. This entire process, including endosperm development, is fertilization independent. Introduction of reproductive tissue marker genes into sexual and apomictic Hieracium showed that AI cells do not express a MMC marker. Spatial and temporal gene expression patterns of other introduced genes were conserved commencing with the first nuclear division of the AI cell in apomicts and the mitotic initiation of embryo sac formation in sexual plants. Conservation in expression patterns also occurred during embryo and endosperm development, indicating that sexuality and apomixis are interrelated pathways that share regulatory components. The induction of a modified sexual reproduction program in AI cells may enable the manifestation of apomixis in HIERACIUM:
Figures






References
-
- Araujo, A.C.G., Mukhambetzhanov, S., Pozzobon, M.T., Santana, E.F., and Carneiro, V.T.C. (2000). Female gametophyte development in apomictic and sexual Brachiaria brizantha (Poaceae). Rev. Cytol. Biol. Veg. Botan. 23, 13–28.
-
- Barcaccia, G., Mazzucato, A., Albertini, E., Zethof, J., Gerats, A., Pezzotti, M., and Falcinelli, M. (1998). Inheritance of parthenogenesis in Poa pratensis L.: Auxin test and AFLP linkage analyses support monogenic control. Theor. Appl. Genet. 97, 74–82.
-
- Bicknell, R.A., and Borst, N.K. (1994). Agrobacterium-mediated transformation of Hieracium aurantiacum. Int. J. Plant Sci. 155, 467–470.
-
- Bicknell, R.A., Borst, N.K., and Koltunow, A.M. (2000). Monogenic inheritance of apomixis in two Hieracium species with distinct developmental mechanisms. Heredity 84, 228–237. - PubMed
-
- Boutilier, K., Offringa, R., Sharma, V.K., Kieft, H., Ouellet, T., Zhang, L., Hattori, J., Liu, C.M., van Lammeren, A.A., Miki, B.L., Custers, J.B., and van Lookeren Campagne, M.M. (2002). Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 14, 1737–1749. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

