Reductive dehalogenation of brominated phenolic compounds by microorganisms associated with the marine sponge Aplysina aerophoba
- PMID: 12839794
- PMCID: PMC165205
- DOI: 10.1128/AEM.69.7.4159-4166.2003
Reductive dehalogenation of brominated phenolic compounds by microorganisms associated with the marine sponge Aplysina aerophoba
Abstract
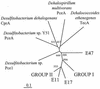
Marine sponges are natural sources of brominated organic compounds, including bromoindoles, bromophenols, and bromopyrroles, that may comprise up to 12% of the sponge dry weight. Aplysina aerophoba sponges harbor large numbers of bacteria that can amount to 40% of the biomass of the animal. We postulated that there might be mechanisms for microbially mediated degradation of these halogenated chemicals within the sponges. The capability of anaerobic microorganisms associated with the marine sponge to transform haloaromatic compounds was tested under different electron-accepting conditions (i.e., denitrifying, sulfidogenic, and methanogenic). We observed dehalogenation activity of sponge-associated microorganisms with various haloaromatics. 2-Bromo-, 3-bromo-, 4-bromo-, 2,6-dibromo-, and 2,4,6-tribromophenol, and 3,5-dibromo-4-hydroxybenzoate were reductively debrominated under methanogenic and sulfidogenic conditions with no activity observed in the presence of nitrate. Monochlorinated phenols were not transformed over a period of 1 year. Debromination of 2,4,6-tribromophenol, and 2,6-dibromophenol to 2-bromophenol was more rapid than the debromination of the monobrominated phenols. Ampicillin and chloramphenicol inhibited activity, suggesting that dehalogenation was mediated by bacteria. Characterization of the debrominating methanogenic consortia by using terminal restriction fragment length polymorphism (TRFLP) and denaturing gradient gel electrophoresis analysis indicated that different 16S ribosomal DNA (rDNA) phylotypes were enriched on the different halogenated substrates. Sponge-associated microorganisms enriched on organobromine compounds had distinct 16S rDNA TRFLP patterns and were most closely related to the delta subgroup of the proteobacteria. The presence of homologous reductive dehalogenase gene motifs in the sponge-associated microorganisms suggested that reductive dehalogenation might be coupled to dehalorespiration.
Figures




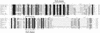
References
-
- Ashworth, R. B., and M. J. Cormier. 1967. Isolation of 2,6-dibromophenol from the marine hemichordate, Balanoglossus biminiensis. Science 155:1558-1559. - PubMed
-
- Baker, J. T., and C. C. Duke. 1973. Isolation from the hypobranchial glands of marine molluscs of 6-bromo-2,2-dimethylthioindolin-3-one and 6-bromo-2-methylthioindoleninone as alternative precursors to tyrian purple. Tetrahedron Lett. 14:2481-2482.
-
- Ebel, R., M. Brenzinger, A. Kunze, H. J. Gross, and P. Proksch. 1997. Wound activation of protoxins in marine sponge Aplysina aerophoba. J. Chem. Ecol. 23:1451-1461.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

