Annexin II regulates multivesicular endosome biogenesis in the degradation pathway of animal cells
- PMID: 12839987
- PMCID: PMC165635
- DOI: 10.1093/emboj/cdg321
Annexin II regulates multivesicular endosome biogenesis in the degradation pathway of animal cells
Abstract
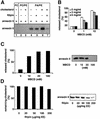
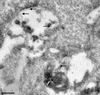
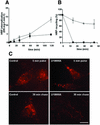
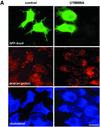
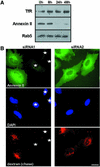
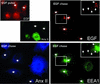
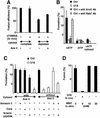
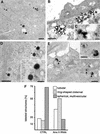
Proteins of the annexin family are believed to be involved in membrane-related processes, but their precise functions remain unclear. Here, we have made use of several experimental approaches, including pathological conditions, RNA interference and in vitro transport assays, to study the function of annexin II in the endocytic pathway. We find that annexin II is required for the biogenesis of multivesicular transport intermediates destined for late endosomes, by regulating budding from early endosomes-but not the membrane invagination process. Hence, the protein appears to be a necessary component of the machinery controlling endosomal membrane dynamics and multivesicular endosome biogenesis. We also find that annexin II interacts with cholesterol and that its subcellular distribution is modulated by the subcellular distribution of cholesterol, including in cells from patients with the cholesterol-storage disorder Niemann-Pick C. We conclude that annexin II forms cholesterol-containing platforms on early endosomal membranes, and that these platforms regulate the onset of the degradation pathway in animal cells.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

